탐방
APRINOIA's different Strategy, expanding 'Tau PET→Therapeutics'
by Sungmin Kim
Tau strategy ① 2nd generation PET tracer ② Three Approaches to Developing Treatment...Strategy for developing Alpha Synuclein PET tracer + treatment

Over the past 20 years, progress in the clinical development of Alzheimer's disease treatments has been in the process of selecting “right patients”. Huge investments were made to focus on the target of amyloid beta (Aβ) in developing treatment for Alzheimer's disease, but several Aβ drugs failed to improve cognitive function. However, with positive news coming out recently, the atmosphere has been reversed. Biogen announced the result of slowing cognitive decline in a phase 3 clinical trial of 'aducanumab', of which approval is to be determined in this June. In January of this year, Eli Lilly's 'donanemab' announced the success of a phase 2 clinical trial. The two clinical trials raised the bar for patient selection criteria in clinical trials. Aducanumab used amyloid PET to select positive patients, and donezumab used both amyloid and tau PET to more accurately screen early patients. In the field of neurodegenerative diseases including Alzheimer's disease, the keyword 'precision medicine' is gradually emerging.
In that respect, the starting point of Taiwan's APRINOIA Therapeutics is unique. APRINOIA was founded in 2015 with a thirst for a new strategy for clinical development. Based on the novel imaging compound discovered by the co-founder Makoto Higuchi, a professor at the National Institute of Radiological Sciences (QST) in Japan, it started as a company that develops the next-generation tau PET tracer, and has expanded to treatments. Unlike other biotech approaches, APRINOIA is unique in that it started with the diagnostics of neurodegenerative disease and expanded the scope of its development to the therapeutic agents.
The start of APRINOIA “Six years ago when the company was founded, we found we might have some a new strategy, and we might have a collection of novel compounds in hand, that we can really contribute to the field.” said Dr. Ming-Kuei Jang, CEO of APRINOIA. “APRINOIA’s tau PET tracer belongs to the second generation and we found one very key difference between our tracer and the others is that not only for AD, but for other rare tauopathies. Our goal is to use the tracer to select patients on what stage of disease to treat with tau, drugs will be more effective and precisely.”
Recently, important milestones have also followed. Last year, the company took a leap toward commercialization as China’s National Medical Products Administration (NMPA) approved a Phase 3 clinical trial of the next-generation Tau PET imaging tracer ‘18F-APN-1607’. Subsequently, in last December, the company signed a non-exclusive license deal for 18F-APN-1607 with Biogen. Also, the company entered into a research collaboration with Insilico Medicine to accelerate the discovery of next generation compounds targeting abnormal proteins in brain associated with neurodegenerative diseases. Based on these capabilities, it will also accelerate the development of new drugs this year. APRINOIA plans to submit a clinical trial plan (IND) for candidate of antibodies and small molecule compounds targeting tau by the end of this year.
Dr. Jang began developing drugs against Alzheimer's disease by discovering drugs at Merck (MSD) in Boston. Then he moved to GSK in Shanghai and was in charge of preclinical research of Alzheimer's disease treatments. At that time, GSK was carrying out a project that invested 100 million US dollars as it moved its R&D hub for neurodegenerative disease to Shanghai. Afterwards, he returned to MD Anderson and participated in a consortium to discover new targets with professor teams such as MIT Baylor School of Medicine and MD Anderson, and later he decided to start a business.
APRINOIA has received a total of $17.5 million through seed investment and series A and B rounds of funding so far. Series B was led by the Korean VC KTB network and participated by China's ShangPharma, Japan's DCI Partners, and Taiwan's TaiAn Technologies.
'Patient Selection'
In the early-mid 2010s, around the time APRINOIA was established, antibodies targeting amyloid beta, such as Roche's 'Gantenerumab', Pfizer-Jansen's 'Bapinezumab', and Lily's 'Solanezumab', reported successive clinical failures. At that time, the industry felt that a breakthrough was needed, and there was a thirst for new drugs and clinical strategies.
“What we have observed from the failure of the previous amyloid trials, is a lot of clinical studies patients may not well selected. A lot of patients were actually at mid-stage or late stage of patients of neurodegeneration, which is a very complicated disease.” said Ming-Kuei Jang.
Early diagnosis was the key to selecting right patients with mild cognitive impairment (MCI) or earlier stage for drug development and even for better treatment of the diseases. In addition, as a new clinical development strategy in 2016, Biogen in the aducanumab clinical trial selected patients based on the results of amyloid PET imaging and announced the results of the phase 1b clinical trials that aducanumab delayed cognitive decline. The result got a positive evaluation, showing that in early stages it demonstrated therapeutic potential. Last year, Biogen released data that delayed cognitive decline in only one of two independent phase 3 clinical trials, and based on this result, it is pursuing approval for the first Alzheimer disease treatment.
“There are a lot of good tools and also drugs targeting beta amyloid. We can see some antibodies developed by major pharmas, they cannot reduce beta amyloid in patience very effectively, so as to their cognition is not improving. That's the key failure for amyloid field,” he added. “Now whether beta amyloid hypothesis is correct or not, if we hope that beta amyloid therapy can be affected on every stage of patients, you have to treat beta amyloid as early as possible, because beta amyloid accumulation occurred really early, maybe 10, 15 years before you have symptoms. It's hard to treat those people without symptoms, but I'm still hopeful for amyloid hypothesis, and selecting right patients matters."
APRINOIA also focuses on early diagnosis. Dr. Jang noticed a unique library of compounds, discovered by Dr. Makoto Higuchi, which appeared to specifically recognize the pathological form of tau protein in the brain. Dr. Jang signed a license and strategic partnership with the research team led by Dr. Higuchi and then established APRINOIA in 2015. It is also important that PET imaging is the only way to measure specific proteins in the brain. "The brain is a separate organ and can’t be examined by way of punctuation, and biopsy is difficult," said Ming-Kuei Jang. "Imaging is the only way to see what's going on in the brain.”
Why tau?
APRINOIA has been focusing on developing tau imaging PET tracers and therapeutics for many years. "Tau is a relatively younger field," said Ming-Kuei Jang.
APRINOIA’s view about tau proteins in neurodegenerative disease is clear. "When tau aggregate appears, you expect see a neural degeneration affecting mind and behavior. So, it looks like tau aggregation is the trigger of the disease and also the trigger of cell death. Our idea is that if you can remove the trigger, tau aggregate, even if you have beta amyloid appears in the brain without the trigger, cell death may not happen.”
The progression of AD is associated with the location and level of accumulation of abnormal tau proteins; therefore, it’s helpful for doctors to estimate the development of neurodegeneration for patients which parts of the brain will be affected in 3 to 5 years. That is why tau can be used as a biomarker to diagnose pathological stages. In addition, when mutations such as P301L, K257T, and S305S occur in the MAPT gene encoding tau, patients suffer from Frontotemporal dementia (FTD). In PSP (progressive supranuclear palsy) patients without genetic mutations, only tau aggregates are seen in the brain. Both diseases are classified as primary tauopathies in the sense that tau is a major factor causing stage progression. This is why tau protein is viewed as a promising therapeutic target in the field of neurodegenerative disease.
Tau is a large and complex protein compared to amyloid beta. Amyloid beta is made up of 36 to 43 amino acids. On the other hand, in human brain, tau protein has 6 isoforms, in which3R tau or 4R tau have different expression patterns in tauopathies respectively. This is one of the traits to differentiate tauopathies by identifying 3R or 4R tau aggregates. The tau protein is composed of 351 to 441 amino acids, depending on the result of gene splicing (MAPT splicing).
Under normal conditions, tau maintains the axon function within neurons and is soluble. However, due to probable genetic mutation, the abnormal tau protein starts aggregate. At this time, the types of pathological tau are different in tauopathies relatively. For example, in the brains of Alzheimer's disease, both 3R and 4R tau aggregates are founded, whereas in progressive supranuclear palsy (PSP), a rare neurodegenerative disease, only 4R tau accumulations are seen, while Pick's disease is found only 3R tau aggregates.
This adds complexity. "The cell type of tau aggregates are different depending on the disease," he said. "In Alzheimer's disease tau aggregates are mainly accumulated in neurons, but in PSP, they are mainly accumulated in astrocytes. Also differences depending on the stage,” he added.
Tau strategy ① 2nd generation PET tracer
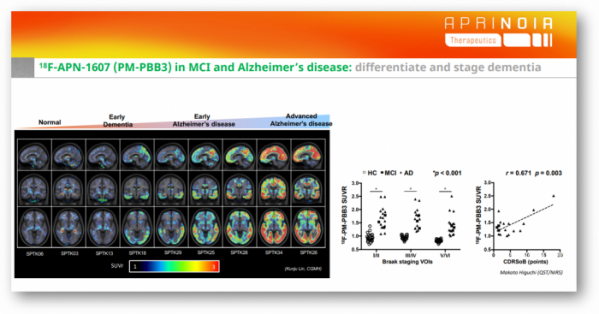
APRINOIA's goal is to select right patients with specific diseases and to identify stages by imaging tracers for more effectively and precisely disease management, as well as for clinic trials when developing treatments. APRINOIA's tau PET tracer ‘APN-1607(18F-PM-PBB3)’ is the second-generation tau PET tracer with better specificity on 3R and 4R tau, which differentiates itself from its competitors in that it can accurately diagnose not only Alzheimer's disease but also other rare tauopathies.
Lily's TAUVID™, flortaucipir F18, a first-generation tau PET tracer first approved last year can detect tau in the brain of Alzheimer's disease patients, but not tauopathy, where only one pathological protein of 3R or 4R tau is prominent. The drug binds to the 3R/4R tau helical structure (PHF) seen in Alzheimer's disease patients, but the binding force is weak on 3R and 4R tau. In addition, tauvid lowered the diagnostic accuracy due to off-target issue where tauvid binds to other proteins in the brain (MAO-A, B) as well as tau.
In an attempt to overcome these limitations, there is a second generation Tau PET '18-F-PI-2620'. PI-2620 is a drug developed jointly by Life Molecular Imaging and AC Immune. It is a drug that targets 'best-in-class' because it can bind to 3R and 4R tau. Last year in JAMA Neurology, they announced the possibility of diagnosing PSP patients with 4R pathological protein.
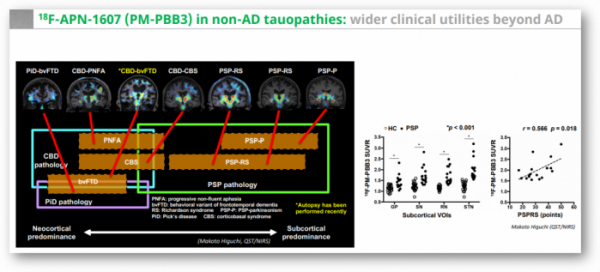
APRINOIA went one step further. The research team of QST led by Dr. Makoto Higuchi, a co-founder of APRINOIA published findings at Neuron that 18F-APN-1607 (18F-PM-PBB3) screened and diagnosed Alzheimer's disease and FTLD, including Corticobasal degeneration (CBD), PSP, and Pick's disease with high-resolution images (doi: 10.1016/j.neuron.2020.09.042). In the paper, Makoto Higuchi's research team added, "its capability to sensitively visualize FTLD-spectrum tau pathologies has been controversial because of a lack of compelling evidence for the agreement of radioligand retentions with PSP, CBD, and PiD tau topologies."
APRINOIA is conducting clinical development of 18F-APN-1607 targeting about 700 subjects in different programs. Specifically, they are conducting phase 2 clinical trials in the US for non-patients, patients with mild cognitive impairment (MCI) and Alzheimer disease patients (NCT04141150). They are also conducting phase 2 clinical trials in Taiwan and phase 1 clinical trials in Japan. Phase 3 clinical trials in China, which are the most advanced in development, are expected to begin mid-this year. Partnerships in other regions, including Korea, are always open to consideration.
In addition, it is developing 'APN-1701'as an advanced tracer of 18F-APN-1607, where the former improves the need for a specific yellow light when manufactured. The function of the compound binding to tau is the same, and only the light sensitivity is improved.
If the tau PET tracer is released, wouldn’t it be limited to use in the clinical field, due to the fact that there is no effective treatment for tauopathies, the cost of screening is high while insurance is not covered?
Dr. Jang said, “In terms of diagnosis, the brain is a different organ. For identifying neurodegenerative disease, location matters. Tau PET imaging is the only way to see tau aggregate in different locations of the brain. Different location controls different phenotypes Therefore, looking for where tau aggregate starts in brain and also where the neurodegeneration starts, where the generation spread will be important to differentiate whether this patient is early mid stage or late stage. Now it looks like only tau PET imaging can achieve accurate results.”
Tau PET tracer has these characteristics, first, tau PET can be used to select patients with early Alzheimer's disease in clinical practice. If we just use Amyloid plaque as the only criteria of selecting patient it will be a bias because Amyloid plaque maintains the same level in the mid- and late- stage of AD. On the contrary, the degree of accumulation of tau proteins varies depending on the stage of the pathology, allowing early patients to be selected. Selecting right patients can benefit to drug development for neurodegenerative diseases. Second, it can be used for evaluating candidate molecules’ efficacy by tracking changes of tau biomarkers in the brain.
In addition, APRINOIA is also attempting to develop an ELISA-based blood test that measures tau pathology in the blood in the long term. "If we can detect aggregated tau in blood in the future, it will be possible to first select positive patients through large-scale screening and then conduct PET imaging tests," said Dr. Ming-Kuei Jang. "Blood test and PET imaging can complement each other.”
In actual clinical practice, there were examples showing the importance of developing PET tracers that can be applied not only to Alzheimer's disease, but also to various tauopathies. As a clinical development strategy chosen by big pharma companies when developing tau antibodies, it is first verified in the PSP, where pathological tau is a problem, followed by clinical development for Alzheimer's patients with complex pathological mechanisms. It is a concept that first secures proof-of-concept data in diseases with a relatively simple mechanism of action. In addition, PSP is a rare disease that affects 1 in 100,000 people, and has a regulatory advantage when developing new drugs.
However, AbbVie and Biogen, who were developing tau antibodies with this strategy, announced that they did not see a difference in efficacy compared to placebo in the phase 2 clinical trial for PSP in 2019. At the time, the industry mentioned the possibility of the drug efficacy of tau antibodies or the selection of tau epitopes in PSP patients with relatively rapid staging as the cause of clinical failure.
Dr. Jang interprets the result as a problem of “patient selection.” Because there is no tau PET tracer that can diagnose PSP patients, clinicians select patients based on symptoms. However, he explained that it is difficult to accurately diagnose PSP in early patients. “From our experience of image studies, we found half of the patients of selected are not PSP,” he said. “When the patients are still at early stage of PSP, they look very similar to Parkinson’s disease. Clinicians cannot tell the difference between Parkinson’s disease and PSP only when the PSP patient is more advanced can be differentiated. Some failed clinic trials might be because of wrong selection of patients.”
As another possibility, he pointed out that the mechanism by which tau proteins leave neurons and spread differs depending on the disease. Tau begins to aggregate within nerve cells, is released as the cells die, and spreads to other areas. Therefore, the approach to preventing the transmission of tau is mainly used for antibody treatments.
"There is strong evidence that tau can be transmitted in Alzheimer's patients and spread to neighboring neurons," he said. “However, we don’t know exactly at which stage the tau transmission occurs about the PSP disease, or the tau may not be released.” It means that patient selection in clinical design must be reconsidered, and it is still a promising approach.
As a result, the advantage of 18F-APN-1607 tracer of APRINOIA can be highlighted in that it can distinguish PSP from other tauopathy patients. In fact, Biogen, which signed a non-exclusive license agreement with APRINOIA for the tracer last year, is also in the process of clinical development of three new drug candidates for tau, so it seems that the tau PET tracer of APRINOIA is attractive.
Tau Strategy ② Three Approaches to Developing Treatment
PET tracers have a pharmacokinetic (PK) feature that rapidly penetrate into the brain across the blood-brain barrier (BBB) and bind to tau, and then the tracers detach with tau and then exit quickly. There is a concern that the image resolution decreases as non-specific binding increases. Then, would it be possible to develop a drug that penetrates the BBB and binds to tau for a long time as a treatment? Considering that PET tracers have the characteristics of binding to tau aggregates, APRINOIA develops the tracer with different PKs.
A company showing a strategy similar to that of APRINOIA is AC Immune, based in the US, which focuses on neurodegenerative diseases that have partnerships with big pharmas such as Lily, Genentech, and Janssen. AC Immune has been developing antibody therapeutics and PET tracers targeting pathological proteins such as tau, alpha-synuclein, Aβ, TDP-43, and inflammasome, and recently SM-based inhibitor 'Morphomer,' based on a small molecule compound library. It has expanded its scope to a platform. The most advanced project is the Tau Morphomer program (Tau Morphomer), which has signed a big deal totaling over $2 billion, including a down payment of $80 million with Lily.
"APRINOIA has the technology and know-how to modify binders to change those PK profiles," said Ming-Kuei Jang. "So in the past of five years, we had spend a lot of time, investing, understanding the SAR, structure, activity, and relationships. Now we have more than 1,500 compounds surrounding the pockets.”
APRINOIA develops tau treatments from multiple angles. Tau aggregates accumulate in neurons andbecome toxic and kill cells. Then these "bad" aggregates are released outside the cell and enter other neurons. In other words, tau begins in a confined area like an infectious disease and spreads to other parts of the brain. “To target various types of tau, drugs of different strategies are needed.” added Ming-Kuei Jang.
▲ Small molecule (SM)-based inhibitors targeting intracellular aggregates ▲ Antibodies that trap tau that propagates to other cells outside the cell and prevent the progression of the stage ▲ Degrader using a protein degradation system, etc.
"If you want to target those different types of tau, maybe you need to have different molecules. Tau antibodies strategy is mostly to a keep the disease at that stage, without getting worse further; while small molecule drugsyou can actually clear much more tau aggregates," he said. "we're thinking about using both small molecules and antibodies to target different pathologies in the future. Either with antibodies, or small molecules, or combine the both, for us, we want for all the possibilities.”
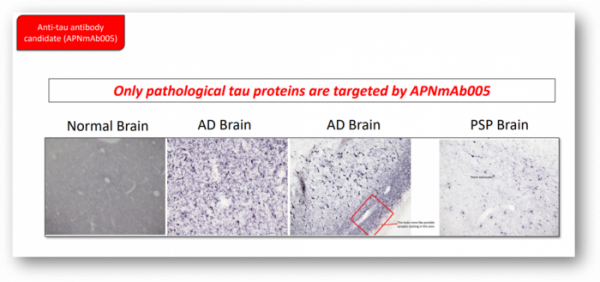
One of the most advanced project is 'APNmAb005', a tau antibody. APNmAb005 was described as an antibody that specifically recognizes the 3D conformation of pathological tau aggregates observed outside the cell. "The antibody is a disease-specific antibody," he added, "It's a very unique epitope that does not overlap with other competitors.”
In actual brain samples of normal people, Alzheimer's disease patients, and PSP patients, APNmAb005 was used to determine the location of the pathological tau. From the results, it’s clear that APNmAb005 is only targeting pathological tau proteins, showing the sign of blocking the abnormal proteins spreading. In addition, the pattern of pathological tau was different depending on the disease. It was detected near the synapse of neurons in the cerebral samples of Alzheimer's disease patients, and the brain tissues of PSP patients were specific for astrocytes.
'APN-729' is the next promising small-molecular compound of inhibitor project. "They're mostly purely binders," said Ming-Kuei Jang "Our idea is to prevent further aggregates from forming as small molecules bind to tau aggregates, or to change the balance of different tau species, or block downstream effect.”
Finally, a degradation drug targeting intracellular aggregation tau is also being developed. APRINOIA is developing decomposition drugs using the ubiquitin proteasome system (UPS) and autophagy decomposition system.
APRINOIA believes that both of the ubiquitin proteasome system and the autophagy decomposition system approaches are necessary to eliminate many types of pathological tau aggregates. "Different forms of tau aggregates will need different trash cans," he explained. "For example, for large aggregates, I don't think proteasome system will be sufficient because you need to open up the protein and then put proteins through the proteasome. But for autophagy, in the cell membrane, it ‘eats’ a lot of hardcore, large tau aggregate. Therefore, I think both systems will have different utilities.”
Because APRINOIA has designed a binder that binds to the tau protein, it can add elements such as a ligand that binds to E3 ligase. Currently, proteolytic drug programs using proteasomes are leading, and strategies using autophagy are in the early stages.
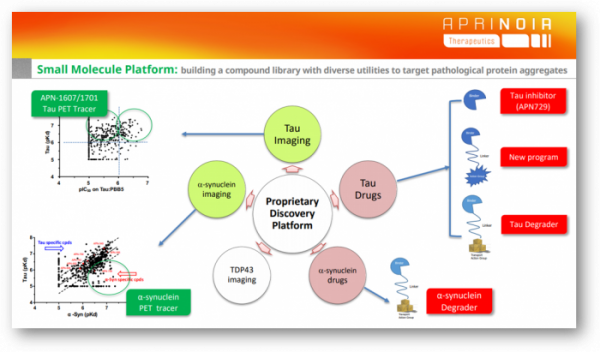
Strategy for Alpha Synuclein
The pathological protein that APRINOIA targets after tau is α-synuclein (α-Syn). Alpha-synuclein also leads the development of PET tracers, followed by a strategy to develop therapeutics.
In the field of neurodegenerative diseases, PET tracers targeting amyloid and tau are actively developed, but Alpha-synuclein has a big challenge, so only a few companies develop it. As the leading company, AC Immune is in non-clinical developments such as ACI-12589 and ACI-3847. “There's a critical need for imaging but now the development of the tracer is very challenging for Alpha-synuclein, So when you look at the clinical progress, there's none, unfortunately.” said Ming-Kuei Jang. “We can recognize the unmet needs. Therefore, we decide to invest in Alpha-synuclein and found some binders of it for drug development.”
APRINOIA received a research funding for the development of α-Syn PET tracer from the Michael J. Fox Foundation (MJFF) in 2019 and has continued the partnership.
There is more scalability. α-Syn PET is applicable not only to Parkinson's disease, but also to various dementia diseases with alpha-synuclein pathology such as Lewy body dementia and multiple system atrophy (MSA). Like tau PET tracer, it can be applied to diagnose these patients more precisely.
"There is strong evidence that alpha-synuclein causes pathology in these diseases," he said, "In Asia, the most prevalent dementia is AD. The second is a Lewy body dementia. Alpha-synuclein is driving Lewy body dementia, as the same condition of the other neurodegenerative diseases, I think that the earlier diagnosis is made, the higher the likelihood of treatment.”
However, in the α-Synpathologies field, APRINOIA targets small molecule compound-based drugs with fewer competitors than antibody modality. APRINOIA is developing small molecule compounds or degradation drugs that inhibit aggregates based on the α-Syn binder library.
Lastly, regarding future IPO plans, Dr. Ming-Kuei Jang said, “Now we are planning to go to an equity market, but we really haven't decided which market we will pursue. We're evaluating all the possibilities.”
“APRINOIA is getting into commercialization for the tau PET tracer, we're getting into China market first, then to the US market. But for therapeutics, we're targeting the global markets; therefore involving global investors will be important.”





![[인사]한미그룹 2026년 정기 임원인사](https://img.etoday.co.kr/crop/74/74/2071974.jpg)








![[인사]한미그룹 2026년 정기 임원인사](https://img.etoday.co.kr/crop/77/77/2071974.jpg)


