탐방
ViroCure’s Strategic reorganization in two directions for Oncolytic virus
by Sungmin Kim
CEO Park, also as a surgeon, felt 'unmet needs' in the treatment of terminal cancer patients decided to join the firm from his cancer virus research

Oncolytic virus development biotech, ViroCure is about to change its constitution back to a R&D-oriented company. Since June of this year, the new CEO Dong-guk Park , a cancer surgery specialist at Dankook University, has been recruited as a co-representative. In the past, ViroCure was a company that focused on oncolytic virus research, but with the joining of new CEO Park, the weight of each oncolytic virus project's clinical development strategy is increasing. In line with these changes, the final results of the Australian phase 1b clinical trial for PD-1 co-administration from the lead project of the oncolytic virus will also be drawn at the end of this year .
After obtaining a doctorate in medicine from Seoul National University, Park served as the dean of the Graduate School of Medicine at Dankook University, the Biobank of Dankook University Hospital, and the general manager of the NGS Center. In addition, last month, Seoul National University Professor Cho Nam- hyuk was recruited as the Chief Scientific Officer (CSO). CSO Cho is an expert in immunology, microbiology, and vaccine development for infectious diseases, and conducts next-generation oncolytic virus research .
The reason why CEO Park, who is an authority on colorectal cancer and peritoneal cancer surgery in Korea, decided on a new drug development biotech as his new destination was the unmet medical need he felt desperately while treating terminal cancer patients.
CEO Park has actively performed HIPEC procedure to treat cancer patients with peritoneal metastasis since 2010s and contributed to establishing itself as one of its treatments. Peritoneal metastases occur in the late stages among cancer metastases, and are usually targeted for patients with advanced colorectal cancer, gastric cancer, and ovarian cancer that have metastasized after undergoing multiple previous treatments, and there are no remaining treatment options at that point. After the HIPEC procedure with successful operation, some patients survived up to several years, but in many cases, the survival period was less than 6 months.
This naturally led to a thirst for new treatments. CEO Park said, “I have been interested in immunotherapy since the time when drugs such as IFN were released, and I had high expectations. However, the actual immune checkpoint inhibitor has limited efficacy in patients. In order to break through these limitations and increase the survival rate of patients, a new treatment is needed, and I joined ViroCure with the expectation that the combination of anti-cancer virus and immuno-oncology could provide the answer.”
The overall R&D strategy is also reorganized. CEO Park said, “If we have been conducting oncolytic virus projects from the perspective of a researcher, now we will establish a development strategy by subdividing the indications to which the oncolytic virus can be applied from the perspective of clinicians. The oncolytic virus research is ultimately developing drugs for patients. We look forward to close cooperation at Dankook University Hospital Cancer Center in the fields of preclinical and clinical development with my onboard.”
In addition to this unmet need, CEO Park's joining ViroCure also played a part in his relationship with the late Professor Man-bok Kim, the founder of ViroCure. In the late 1990s, while he was a guest professor at the University of Calgary, Park met Professor Man-Bok Kim. At that time, Professor Kim was researching reovirus and myxoma virus, which became the starting point of ViroCure, and then invited Professor Kim as a professor at Dankook University to establish an anti-cancer virus center. ViroCure is a company that started as a venture company of Dankook University Industry-Academic Cooperation Foundation in 2016. However, he had no direct relationship with ViroCure until recently, but after retiring this year, while serving as a visiting professor, he became a company advisor and became more deeply involved in the company as he took over as CEO.
When CEO Park was at the University of Calgary, the concept of an oncolytic virus was still unfamiliar. His early attention to oncolytic virus began with a specific phenomenon. First, there was a phenomenon in which tumors have decreased after suffering from a viral infection, and the fact that there were more viruses coexisting with humans than harmful viruses. Next, it was observed that the reovirus proliferated well in colorectal cancer with RAS mutations, bursting the cancer cells (lysis), and did not proliferate well in normal cells. In normal cells, there is a mechanism that prevents the virus from proliferating, whereas in cancer cells, the tumor-suppressor gene is disrupted, allowing the virus to spread more easily.
CEO Park said, “Initially, we focused our research on the ability of viruses to kill cancer cells. Then, with the rapid development of immunology, now the oncolytic virus bursts cancer cells, releasing various tumor-related antigens in the cells, and activating the immune system, such as tumor specific T cells and antigen presenting cells (APCs). ”
In terms of this mechanism of action, it is judged that the oncolytic virus is a good candidate material that can change the tumor microenvironment. However, so far, the anti-cancer virus has been limited in efficacy through monotherapy, and to overcome this, additional virus engineering is needed to improve combination administration with PD-1/L1 immune checkpoint inhibitors or stroma in tumors.
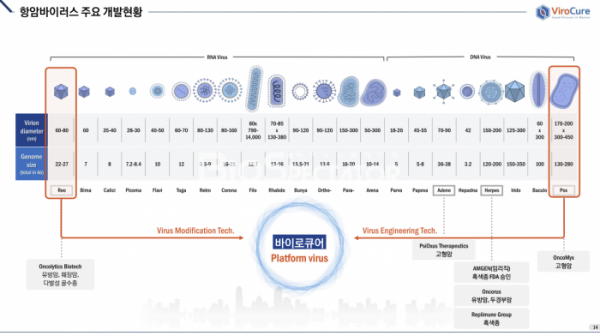
ViroCure also focuses on a combination administration strategy based on Reovirus in the short term and aims to develop next-generation oncolytic virus based on Myxoma virus in the long term. First, the Reovirus is a non-enveloped, double-stranded RNA virus. The virus size is 60 to 80 nm (diameter) and the genome size is 22~27 kb. It is characterized by infection to the respiratory tract and digestive system in the human body.
While developing the wild-type Reovirus RC402, ViroCure is also developing the Reovirus (RP116) that modifies the spike λ2, a binding site recognizable by neutralizing antibodies in the body. RC402 has cleared patent registration in Korea, China and Japan, while RP116 has completed U.S. patent registration, the company said. ViroCure is considering indication of RC402/RP116 for lung and colon cancer, which are respiratory and digestive cancers, as well as carcinomas with high mutations in tumor suppressor genes (p53, ATM, etc.) that show high proliferation of the Reovirus.
ViroCure is in the phase of completing the Australian Phase 1b clinical trial for RC402(IT) in terminal solid cancer as a lead program and is evaluating RC402 monotherapy and Keytruda combination therapy. No dose-limiting toxicity (DLT) was observed until the highest dose (1.4x10^10 pfu) as an interim result so far. The final results of phase 1a/b clinical trials are expected to be available in the second half of this year, and based on these results, company plans to design phase 2 clinical trials. As a competitor, Oncolytics Biotech is conducting development of 'pelareorep (IV)' for co-administration in solid and hematological cancers based on reovirus.
In addition, considering other routes of delivery, ViroCure is also open to the possibility of development as a substance that modulates the intestinal immune response using the characteristic that reovirus infects the digestive tract. The company announced the results of the joint research project with CHA Bundang Hospital to test the development potential of 'RC402-PO', an oral route of administration, as an immunotherapy at the American Association for Cancer Research (AACR).
ViroCure is looking at the possibility of a myxoma virus, a DNA virus, that is easy to engineer due to its large size in the long term. Myxoma virus belongs to the rabbit poxvirus. Since it is not pathogenic in humans and has never been exposed to the human immune system, it can be developed as an intravenous administration route. Viruses are 200-300 nm in size and their genome is 130-280 kb. This has the advantage of being able to load multiple therapeutic genes (transgenes) .
ViroCure has about 10 types of libraries that can be used or installed in combination with the myxoma virus on its own. It is expected that the engineered myxoma virus (MC509-N1) can be developed as a backbone as a next-generation oncolytic virus or cancer vaccine. As a competitor, OncoMyx Therapeutics is developing an intravenous oncolytic virus loaded with IL-12, which activates T and NK cells, and decorin, an immunomodulatory factor in the tumor microenvironment.



















