탐방
Cha Vaccine Institute develops and expands therapeutic vaccines using new immune enhancement platform
by Joungmin Cho
Secure production technology for TLR2/3-stimulating immunity enhancement platforms and recombinant antigens which offer various functional groups

Cha Vaccine Institute adds its own immunity enhancement technology to recombinant antigen production technology used for developing preventive vaccines as part of its effort to develop a cure for diseases. The institute has succeeded in independently developing immune enhancement platform technologies called 'L-pampo' and 'Lipo-pam'. Cha Vaccine Institute's 'Adjuvant' can induce effective immune response by stimulating TLR2 and TLR3 simultaneously. This adjuvant has been proven to be safe for human use through clinical trial.
CEO Yum said "Today, immunotherapy is in the spotlight in various treatment fields, including the anti-cancer field. Adjuvant developed and owned by Cha Vaccine Institute is capable of activating immune response by itself. And It can be loaded with various types of antigens including DNA and RNA. Thus, it has great scalability not only for currently possessed pipelines, but also for various diseases". Cha Vaccine Institute has the potential to become a partner for development of an immune oncology combination therapy, the research of which is now expanding globally.
Immunity enhancement platforms possessed by Cha Vaccine Institute can be divided into two forms: 'L-pampo' which is an immuno-stimulating adjuvant technology and 'Lipo-pam' which is a delivery technology of particle formulation. L-pampo enhances the immune system by targeting TLR2 and TLR3 receptors of antigen-presenting cells that play an important role in immune response. CEO Yum said, "L-pampo' can produce synergetic effects by simultaneously acting on both TLR 2, which exists on the surface of an antigen-presenting cells, and TLR3, which exists inside antigen-presenting cells.
Cha Vaccine's L-pampo consists of bacterial lipoprotein Pam3 and synthetic double-stranded RNA PolyI:C. It first activates TLR2 on a cell surface and then goes into the cell in an enclosed form to stimulate TLR3 inside the cell. TLR3 is a receptor that recognizes double-stranded RNA of infected virus inside the cell. When activated, it stimulates signaling of the antiviral defense system.
CEO Yum said, "We confirmed that when L-pampo was applied, we were able to induce both cellular immune response such as Th1 and humoral immune response such as antibodies." Cha Vaccine Institute conducted a joint research with the research team of Yonsei University to confirm the mechanism and effect of L-pampo. The research results were published the in an international academic journal in 2016.
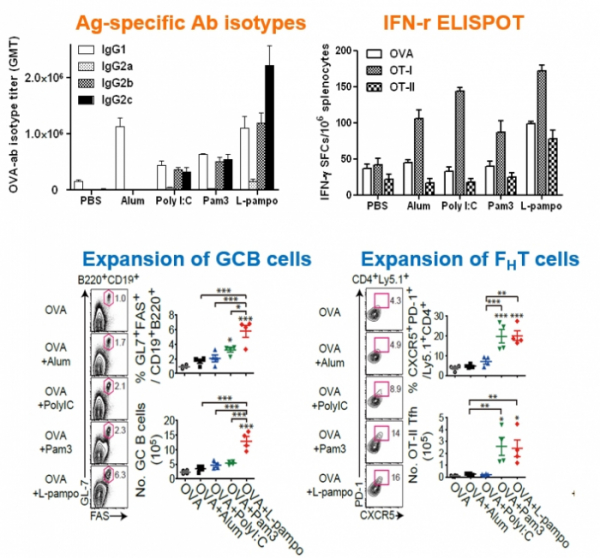
▲MOA(mode of action) of 'L-Pampo'
CEO Yum said, "We have confirmed that our adjuvant shows better immune enhancing effect than existing adjuvants including MPL, CpG, MF59, and Alum. In addition, the application of adjuvant alone can enhance immune responses. When it is applied as a combination therapy with immune checkpoint inhibitors that are now grabbing much attention, a synergetic effect is expected. In fact, Tollbridge Therapeutics in the US has developed 'Diprovocim' that acts on both TLR1 and TLR2 in a similar way to that of Cha Vaccine Institute, and is currently trying to develop a combination therapy of 'Diprovocim' with PD-L1 antibody with a strategy to solve the low response rate of immune checkpoint inhibitors. The US pharmaceutical company has recently attracted a great deal of investment.
The other adjuvant technology of Cha Vaccine is 'Lipo-pam', a technology that delivers a substance in the form of particle formulation. Lipo-pam is in the form of liposome. It can stably deliver antigens consisting of DNA, RNA, and peptides. In an experiment conducted by researchers of Cha Vaccine Institute, it was observed that Lipo-pam showed excellent ability to deliver and express antigens into cells.
CEO Yum said, "We confirmed that Lipo-pam could reliably deliver RNA with a low in-body stability. In an experiment to confirm intracellular delivery, we found that it showed a better or similar delivery ability of RNA compared to Lipofectamine. Lipo-pam has a superior delivery ability to the extent that it is called the next-generation vaccine. The advantage is that it is highly likely to be utilized as a future RNA vaccine platform."
Cha Vaccine is developing vaccines that can prevent and treat diseases by adding antigen recombinant technology to these adjuvant technologies. Its pipeline includes a hepatitis B treatment vaccine, herpes zoster vaccine, geriatric flu vaccine, and anti-cancer vaccine. The fastest developed vaccine is the hepatitis B treatment vaccine (CVI-HBV-002) which is in preparation for Phase 2b clinical trial.
Cha Vaccine has developed a powerful vaccine candidate 'CVI-HBV-002' that can overcome immunity tolerance of patients with chronic hepatitis B by discovering the third-generation antigen with high immunogenicity through its own antigen recombination technology and employing the strong immune enhancing adjuvant L-pampo. The used recombinant antigen provides more epitopes as it has the form of adding antigens of HBV virus to the existing antigen surface. These existing antigens have to be administered three times to generate antibodies. However, Cha Vaccine's antigen need only two times of administrations to form antibodies. It also shows a high antigen potency.
CVI-HBV-002 is a therapeutic vaccine aimed to cure hepatitis B by activating Th1 immune response and ultimately eliminating the virus. In an animal experiment, once CVI-HBV-002 was administered to mice in the state of immune tolerance, in which the immune response would not occur, it produced an antibody against S antigen and dramatically reduced HBV S antigen in the blood.
Cha Vaccine carried out Phase1/2a clinical trials on patients with chronic hepatitis B virus who were receiving oral antiviral drugs in order to assess the safety, tolerability, and efficacy of CVI-HBV-002. The primary evaluation factor was safety. The clinical trial was conducted on a total of 53 patients by dividing them into six cohorts. It evaluated the safety of a new HBV vaccine as a primary endpoint. Any side effect or allergic reaction was not observed. The company stressed that the activation of HBV-specific T cells was inducted in 85% of patients in the state of immune tolerance. These results confirm that the vaccine has potential to cure hepatitis B with a strong immune response hepatitis.
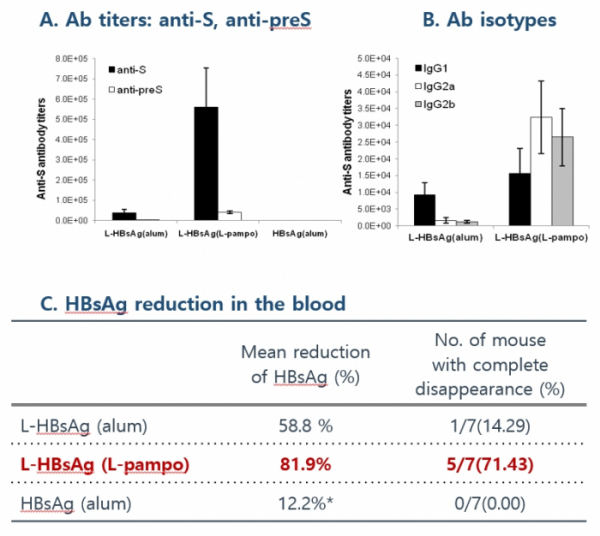
CEO Yum said, "The safety of CVI-HBV-002 was confirmed through Phase 1/2a clinical trial. When clinical results of CVI-HBV-002 were compared to those of Gilead GS-4774, CVI-HBV-002 was found to have better effects. We are currently planning and preparing for Phase 2b clinical trial in the form of expanding the current clinical trial."
In addition to hepatitis B vaccines, Cha Vaccine is developing therapeutic vaccines for herpes zoster, flue, and other serious illnesses that often occur in old-aged group. By strengthening immune responses weakened by aging, it is expected to produce both preventive and therapeutic effects.