탐방
BioSquare’s Innovation of Diagnostic Technology with High Sensitivity ‘Quantum Dot’ Technology
by Joungmin Cho
Multiplex diagnosis with high sensitivity and high precision is possible by the bio-diagnosis platform technology called ‘Quantum PACK’ based on quantum dots, which are ultra-nano semiconductor partic

BioSquare Inc. is developing diagnostic products with high accuracy and sensitivity by integrating quantum dots, a semiconductor material used in electronics, into biotechnology. Use of quantum dots, with sophisticated color reproduction and varying wavelength of light, enables detection of a multiple number of antigens without special amplification, facilitating on-site diagnostics that must be performed quickly and easily.
“We have a strategy to enter the market quickly without the approval procedure for any new medical technology,” said CEO Yoon Sung-Wook, with 19 years of experience in developing various diagnostic technologies and products at LG Chem (former LG Life Sciences) and CJ Healthcare. BioSquare has introduced an on-site influenza diagnosis kit and envisions developing diagnostic products for infectious and chronic diseases.
◇ ‘Quantum Dot,’ nano-size semiconductor particles: excellent color reproduction, excellent light emission, and multiplex-detection.
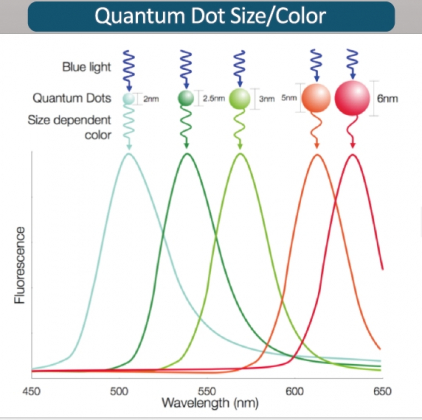
Quantum dots are nanometer-size semiconductor particles with unusual electrical and optical properties. When quantum dots are exposed to light, they emit light of specific frequency. Wavelength of the emitted light may vary depending on the size of quantum dots.
CEO Yoon explained, “Quantum dots can express colors with great detail and in a sophisticated way, and as the wavelength of light may vary depending on the size, it is possible to realize colors that we have not seen before.”
This means that it is possible to detect a multiple number of substances simultaneously by applying quantum dots of various sizes.
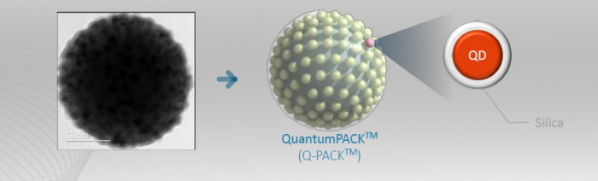
BioSquare developed a platform by introducing the concept of ‘QuantumPACK’ developed by Prof. Lee Yoon-Sik of Seoul National University. The term ‘QuantumPACK’ means nano-size particles coated with silica, raw material of glass, which retains biochemical stability and also has water-soluble properties.
CEO said, “Many different technologies are being developed to package quantum dots. Among them, our newly-introduced silica packaging technology has the advantage of maintaining light efficiency because interference does not occur.”
BioSquare has developed and optimized QuantumPACK by attaching quantum dot to a core comprised of silica of which the outside is covered in silica. The company said that it is possible to detect and amplify a signal hundreds of times only with one particle by packaging 500 quantum dots that can emit strong light into a particle with just one. Additionally, as expressed wavelength may vary depending on size, multiplex detection in a tube is possible.
BioSquare is developing QuantumPACK technology as a product that can replace fluorescent samples currently used at the field site. CEO explained, “Fluorescent samples used in the current immunodiagnostics are essential and require cool storage. However, as the QuantumPACK is covered in silica, its advantage is strong heat resistance.”
He was also confident about multiplex detection. If antibodies targeting different antigens are applied to quantum packs of different sizes, it is possible to detect several different antigens in a tube. CEO Yoon said, “Even the presence of a trace of an antigen can be detected with a strong signal, so it is possible to detect a wide spectrum of antigens. The advantage of QuantumPACK is that it is possible to detect biomarkers present in a large quantity as well as in a trace amount in the body.”
BioSquare deploys a strategy of entering the market with two types of platforms: ‘QuantumPACK Pro’ and ‘QuantumPACK Easy’. Quantum Pack Pro is a miniaturized version of ultra-precision high-sensitivity equipment used in hospitals, which may be used in emergency rooms and airports, for example, requiring accurate diagnosis in the field site. QuantumPACK Easy uses the same test principle as a reagent for pregnancy diagnosis, which can be used for diagnosing influenza infections.
The fastest developing product is the influenza infection diagnostics product through the QuantumPACK Easy platform, and an experiment is underway to compare its performance with previous approved products. CEO Yoon said, “The experiment results show that the product has a outstandingly high sensitivity similar to the real-time PCR technique. BioSquare plans to conduct clinical trials at two hospitals with a goal of obtaining approval in the third quarter of 2020.”
BioSquare is targeting the developed market first with its high-performance products, and plans to enter developing countries’ markets including Africa through the Official Development Assistance (ODA) programs of national and non-governmental organizations.


















