기업
AItheNutrigene, ‘Paper Biochip’ New Approach of Self-Rapid Diagnosis
by Yoonseok Suh
Combination of ‘accuracy’ of paper biochip-based loop-mediated isothermal amplification (LAMP) molecular diagnosis + ‘convenience’ of colorimetric assay result confirmation..Development of self-rapid

Jongchul Kim, CEO of AItheNutrigene, said “The market for self-diagnostic kits that conveniently diagnose diseases at home is expected to grow enormously in the future. Our goal is to change the paradigm of the self-rapid molecular diagnostics market for various infectious diseases by commercializing a paper biochip-based molecular diagnostic kit using the loop-mediated isothermal amplification(LAMP) method”.
Nutrigene was founded in 2013 by CEO Jongchul Kim, Dr. Jae-sung Lee, and Dr. Byunghun Moon. In the early days, it has been developing health diagnosis solutions through the nutritional supplement business and genome analysis. In particular, the genome analysis and nutritional supplement business served as a cash cow for Nutrigene and has become the foundation for the R&D of diagnostic technology.
Health functional food and genomic analysis business, rapid molecular self-diagnosis kit for infectious diseases, and artificial intelligence-based new drug development business are three businesses of Nutrigene that are a cash cow. Among them, the current focus is on the development of self-rapid molecular diagnostic kits for infectious diseases using paper biochips.
CEO Kim mentioned “We have been developing diagnostic technology based on funds secured through nutritional supplement business and health diagnosis solutions. The COVID-19 pandemic came while we were developing a rapid molecular diagnostic kit that can molecularly diagnose infectious diseases such as Lyme disease prevalent in North America and Zika virus prevalent in Southeast Asia without PCR equipment”.
Nutrigene is currently developing a rapid molecular diagnostic kit that can be conveniently used at home using paper biochips to be applied to the diagnosis of COVID-19. The technology applied to the rapid molecular diagnostic kit is the loop-mediated isothermal amplification(LAMP) method of non-instrument nucleic acid amplification technology (NINA). It is a method of diagnosing disease infection by amplifying nucleic acids at a certain temperature and combining them with fluorescent substances.
Until now, a portable fluorescence detection device is required, but development is in progress to confirm the results by colorimetric assay. If the development is successful, a rapid molecular diagnostic kit that combines the accuracy of molecular diagnostics with the convenience of confirming results through colorimetric assay will be released.
Also Nutrigene merged with AI company 'AIThe' last year and changed its name to AItheNutrigene. AIThe has base technologies such as artificial intelligence (AI) image, video identification, voice replication, and big data analysis and processing. After the merger with Nutrigene, based on the base/fundamental technologies, they are developing diagnostic and prognostic analysis models through cancer pathologies images analysis techniques, such as lung cancer, lymphoma, and residual cancer.
Nutrigene plans to raise funds for the development of rapid molecular diagnostic kits by attracting preIPO investment this year and to proceed with a technology special listing next year. Biospectator learned about the rapid molecular diagnostic kit for infectious diseases being developed by AItheNutrigene.
◆ ‘Accuracy’ of molecular diagnosis + ‘convenience’ of colorimetric assay result confirmation..“Development of rapid self-diagnosis kit that can diagnose infectious diseases within 30 minutes and can be easily used at home”
Sungkweon Kim, managing director of Nutrigene, said “We have currently established a paper-chip-based molecular diagnostic technology and are advancing it so that the results can be easily checked with colorimetric assays, as easily as a pregnancy test. The development of colorimetric assay technology is almost final. Once completed, it will be possible to easily diagnose at home for infectious diseases such as Lyme disease, dengue fever, Zika virus, and tuberculosis, including COVID-19”.
In terms of COVID-19, on-site immunodiagnostic methods, including the existing rapid antigen diagnostic kit, have relatively low accuracy, such as sensitivity/specificity, compared to molecular diagnostics. Therefore, there is the inconvenience of reconfirming the result with molecular diagnostics of RT-PCR even if the result is positive. Furthermore, it has a disadvantage that it is almost impossible to diagnose early-stage and asymptomatic patients. The Ministry of Food and Drug Safety suggested that as a guideline for antigen self-test kits “it should be used only as an auxiliary means due to the drawback of low sensitivity, and if symptoms are suspected, genetic testing is in principle.
In addition, it is explained that diagnostic kits for Lyme disease, dengue fever, and Zika virus are mainly tested using antigen-antibody diagnostic kits, or molecular diagnostics in laboratories.
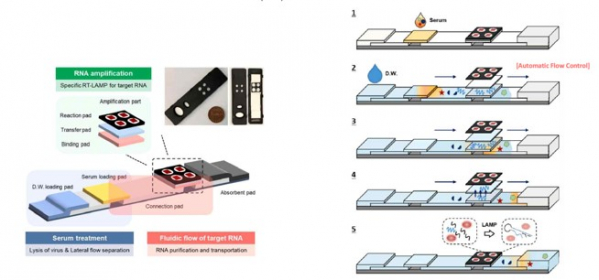
On the other hand, the rapid self-diagnosis kit being developed by Nutrigene gets both the accuracy of molecular diagnosis and the convenience of antigen diagnosis. Nutrigene has △nucleic acid pretreatment technology △loop-mediated isothermal amplification (LAMP)-based multiple diagnostic technologies △paper biochip-based fluid analysis technology.
First, Nutrigene generates a DNA-RNA hybrid through lysis and capturing of a patient's sample, extracts nucleic acids, and combines them with a reaction panel. Next, isothermal amplification detects target genes to diagnose infectious diseases, and multiple targets are diagnosed at the same time using various reaction conditions. In other words, it is possible to simultaneously diagnose COVID-19, influenza A/B, and others with a single sample.
Lastly, the paper chip-based fluid analysis technique is a key technology for low-cost + high-speed, high-sensitivity on-site diagnosis. By implementing both nucleic acid pretreatment technology and isothermal amplification technology on this paper chip, it enables rapid and accurate diagnosis of diseases in the field or at home.
When a sample is loaded on this paper biochip and a few drops of water are added, the water is absorbed into the paper chip and the nucleic acids in the sample bind to the reaction panel. After that, the gene is amplified through isothermal amplification and a fluorescence signal is emitted to diagnose disease. The required time is about 30 minutes with 98% or more of the sensitivity and specificity. No separate analyzer is required, and the price is as low as $13. In the case of COVID-19, it is possible to diagnose early-stage infections and even those who are asymptomatic.
Nutrigene is currently further developing its technology to confirm the fluorescence reaction with a colorimetric assay, the same way that used in pregnancy tester. In other words, it amplifies the target gene by molecular diagnostic method and makes two lines visible if the test result is positive and one line if the test result is negative.
Director Kim said “Since most of the infectious diseases are genetically analyzed, there is expandability to develop a self-rapid molecular diagnostic kit that can easily diagnose not only Lyme disease and Zika virus but also hepatitis B (HBV) at home”. Nutrigene plans to proceed with the development of a diagnostic kit that applies this technology to Zika virus, Lyme disease, hepatitis B (HBV), and even microscopic residual tumors when the development of the COVID-19 rapid molecular diagnostic kit currently being developed is completed.
The competitor is Lucira Health of the United States, which released an all-in-one test kit for diagnosing COVID-19 with emergency approval from the US Food and Drug Administration (FDA) in April. It is a molecular diagnostic method of isothermal amplification using microelectromechanical systems (MEMS) and is used for self-diagnosis on-site and at home. The test takes about 30 minutes. A separate analyzer is not required, but a detector to check the result with fluorescence must also be purchased, and the price is about $50.





![[인사]유한양행, 2026년 1월 임원 인사](https://img.etoday.co.kr/crop/74/74/2044634.jpg)













