기업
Macrogen found New biomarker for Lung Cancer
by Jongwon Jang
Cooperative study of Macrogen, Seoul National University Bundang Hospital and Seoul National University Hospital
A team of researchers at Macrogen and Seoul National University Hospital (Bundang) in Korea has discovered a new biomarker through Next Generation Sequence Analysis (NGS) to find patients suitable for lung cancer immunotherapy. They discovered the ‘M2 Macrophage’, which explores genetic mutations and gene expression, and activates cancer cell proliferation. The researchers expect that the new marker could complement the immune checkpoint inhibitor PD-L1 marker.
According to Macrogen’s announcement on the 9rd, a collaborative team of Seoul National University Bundang Hospital Precision Medicine Center (Research director, Prof. Jungseon Seo), Seoul National University Hospital (Thoracic surgery, Prof. Young Tae Kim) and Macrogen (Senior Researcher, Jong-Yeon Shin) confirmed that the NGS genome analysis method can read immune signals of the tumor microenvironment around tumor cells. Based on these results, they proposed a method of screening patients for whom immune cancer treatment of squamous cell carcinoma of the lung will be effective.
The study was published on the Internet page of the international Journal of American Association for Cancer Research (AACR), Cancer Immunology Research (IF 8.284) on the 2nd May.
The research team extracted DNA and RNA from squamous cell carcinoma tissue of 101 lung cancer patients in Korea, analyzed the results with NGS technology, and examined genetic mutation and gene expression patterns. Of the 101 patients, 82 (81 %, group A: immunodeficient group) had a pattern similar to the known cancer group; but the remaining 19 patients (19 %, group B: Immune hyperactive group) showed a similar pattern to normal people. Group A had more mutation than normal, while group B had fewer mutations, and only immune related genes were overexpressed.
The team backtracked the immune cells with known data and algorithms to see what kind of immunes were involved in the overexpressed genes in the B group. They found that M2 macrophages had been activated. (Macrophages are immune cells, which although a type of leukocyte, produce cytokines that promote cancer growth, and are known to help cancer cells grow).
This means that macrophages lose their function as immune cells due to somatic copy-number alteration (SCNA), and gather around cancer cells to form tumor microenvironment (TME). The researchers found that immune-cancer drugs are effective for patients exposed to these conditions. Based on this result, they devised a diagnostic method that can easily select patients who are effective in immunotherapy.
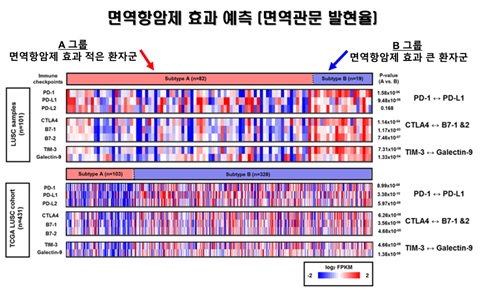
▲한국인 101 명의 폐 편평상피세포암 조직에서 PD-L1과 같은 여러 면역관문 바이오마커들의 RNA 발현량을 확인한 결과, 면역에 따라서 그룹화된 A와 B 사이에 통계적으로 면역관문 유전자 발현량에 유의한 차이가 있음을 확했다. 또한, 암 유전체 지도(TCGA, The Cancer Genome Atlas) 데이터베이스를 통해 431명의 폐 편평상피세포암 환자에서도 이와 같은 패턴이 있음을 입증해 면역항암제에 효용성이 높은 환자군을 식별하는 새로운 바이오마커로서의 가능성을 제시했다. 마크로젠 제공.
In the case of immune-cancer therapy, the biomarker discovery has been an important issue, because even though the therapeutic effect is good, the number of affected patients is limited. The ‘PD-L1 expression rate’ is the best-known biomarker for evaluating the efficacy of immune checkpoint inhibitor; and it is considered that the higher the incidence, the better the treatment effect. However, the limitation has been pointed out that even if the incidence rate is low, there are still patients who respond to immunotherapy, but who are excluded from treatment.
“The new diagnostic test designed this time will complement the limitations of PD-L1 expression. The PD-L1 expression rate is a method of confirming immunological errors based on the protein of the cancer cell itself. The environment is not considered. In contrast, the NGS genome analysis method can detect various immune errors caused by the tumor microenvironment surrounding tumor cells, by the amount of gene expression of the immune cells,” said the research team.
Prof. Seo of the Precision Medicine Center in Seoul National University Bundang Hospital explained, “The results of this study are very meaningful, because it is an alternative presented at a time when the importance of new biomarkers is emerging. The NGS genome analysis method designed through this study is a biomarker that complements the limitations of the PD-L1 expression rate. It can reduce the side effects and costs of unnecessary treatment, and provide treatment opportunities to patients who are excluded from medical benefits.”
The team plans to apply for an international patent on the biomarker discovered. Subsequent studies will also explore whether NGS-based genomic assays can be used as biomarkers for the immunotherapy of adenocarcinoma and pan-cancer in lung. In addition, the researchers plan to develop a vaccine that enhances the effects of immunotherapy using this gene immunity information.













![[인사]종근당 계열사, 2026년 임원승진 인사](https://img.etoday.co.kr/crop/77/77/2277741.jpg)



