기업
SEASUN announces positive results PNA-based eye drops for the treatment of wet macular degeneration
by Jongwon Jang
Non-clinical evaluation demonstrates superior efficacy compared with Eylea, the existing standard injection treatment

SEASUN THERAPRUTICS announces positive results from a non-clinical evaluation of PNA-based eye drops for the treatment of wet macular degeneration.
Non-clinical evaluation demonstrates superior efficacy compared with Eylea, the existing standard injection treatment.
SEASUN THERAPRUTICS disclosed non-clinical evaluation results of POL101, PNA-based eye drops for the treatment of wet macular degeneration. POL101 is an eye drop medication made with POLIGO™, a platform technology of SEASUN THERAPRUTICS, which aims to maximize PNA drug delivery efficiency into tissues like the eye, skin, and brain, and it is a new antisense-oligonucleotide (ASO) drug candidate targeting the VEGF-A gene which contributes to wet macular degeneration.
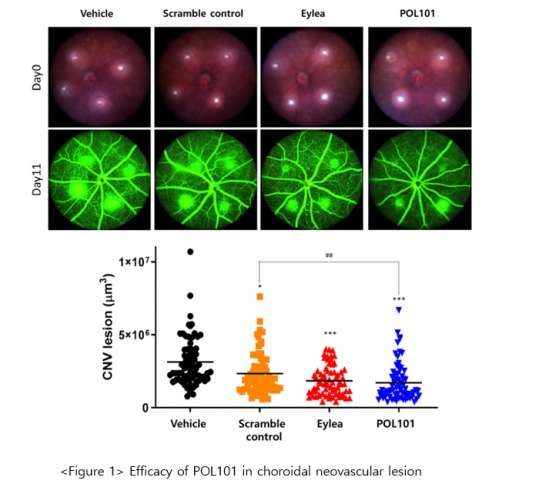
This study was performed to confirm the pharmacological efficacy of POL101 using laser-induced choroidal neovascular (CNV) mouse model for wet macular degeneration. In the POL101-treated group (eyedrop type), the CNV lesion was significantly improved compared to that treated with Eylea (Figure 1. In addition, the expression of the target gene, VEGF-A, was effectively suppressed in the POL101-treated group compared to that treated with Eylea and that normal mouse (Figure 2).
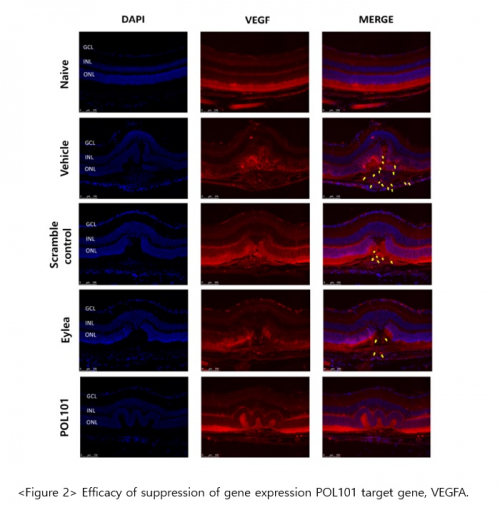
In addition, the expression of angiogenesis factors (ICAM-1, MMP2/9) was effectively inhibited without any differences among groups, and reduction of apoptotic cells in laser-induced choroid neovascular lesions was confirmed.
The publicly announced, non-clinical result was conducted in T2B Infrastructure Center for Ocular Disease in Inje University Busan Paik Hospital, designated by Korea’s Ministry of Health & Welfare.
Wet macular degeneration is a disease that develops choroidal neovascularization in the macula, and tends to get worse with age, causing vision loss. It is the most common cause of vision loss in adults over the age of 60 in developed countries including South Korea, affecting 10% population of aged 66 to 74 and 30% of the population of aged 75 to 85. Conventional wet macular degeneration treatment includes Eylea and Lucentis injections, which are anti-VEGF-A targeted antibody drugs. This means that patients that have to receive these injections must visit a hospital on a regular basis.
POL101 (a wet AMD lead compound), developed by SEASUN THERAPEUTICS, surpassed Eylea in a non-clinical study. A major advantage for patients is the ability to apply the therapy by themselves because it is an eye drop. Therefore, when POL101 gets fully developed and ready for the market, it has a potential to be an innovative drug that can be easily applied and reduce side effects that can occur due to an intraocular injection.
Also, since SEASUN THERAPEUTICS are in process of developing eye disorders pipeline, not limited to wet macular degeneration, including dry macular degeneration (dry AMD), diabetic retinopathy (DR), diabetic macular edema (DME) and so on, it seems that the recent efficacy results can accelerate drugs development for eye disorders. SEASUN THERAPEUTICS would perform the non-clinical study on eye disorder pipelines in a consecutive order.
Hye Joo Kim, CEO of SEASUN THERAPEUTICS, stated that, “We aim to license out to global pharmaceutical companies through POL101 development.” SEASUN THERAPEUTICS is a drug development company diverged from SEASUN BIOMATERIALS, a molecular diagnostic company established in July, 2018.














![[BioS 레터]무균주사제 공급망 변화와 CDMO 대응](https://img.etoday.co.kr/crop/77/77/2262816.jpg)




