기업
PaxGenBio “PCR Innovation, Shortened Time for Examination at Reduced Cost"
by Jongwon Jang
Developed the PaxView Series based on MPCR-ULFA, the Platform Technology..Certified and acquired CE-IVD Marking

▲PaxGenBio President, Yeong-seok Park
PaxGenBio developed the “MPCR-ULFA” platform technology through innovation of PCR device that enabled a great reduction in time for examination and cost thereof. PaxGenBio Company was founded by President, Mr. Park Yeong-seok, under whose responsibility various products have been developed for diagnostics in LG Life Science, Green Cross MS, and Bionia during the past 25 years.
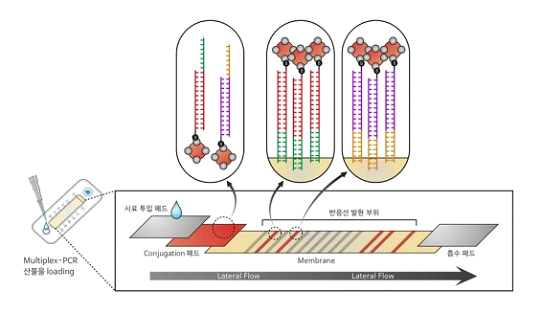
MPCR-ULFA technology was originated from an idea of the addition of single strand probe to the primer used at the stage of amplification of nucleic acid extracted from samples. The universal probe, made contemporaneously during the process of amplification of nucleic acid targeted for detection, combines complementarily with the probe fixed on NC membrane of the kit developed by PaxGenBio and indicates the detection signal.
President Park emphasized, “... The primer varies depending on target nucleic acid; however, the single strand probe, which is used for the detection of nucleic acid, can be employed for universal purposes with high efficiency of reagent used for the detection and with competitive price for fabrication in the form of a kit.
By falling down the outcomes, that underwent the process of amplification of nucleic acid, onto the kit included in the product of PaxGenBio, users can see the results of detection of nucleic acid without going through special processes as involved in an electrophoretic gel. The entire procedure takes only 10 minutes to visualize the results instead of 2 hours required by existing approaches.
PaxGenBio employed this platform technology and developed the PaxView, the quick and convenient molecular diagnostics product, capable of doing genotypic analysis and visually detecting the presence of infection. Among them, the “Paxview-HPV 16/18/Others”, which is capable of performing contemporaneous genotypic analysis of high-risk viruses of cervical cancer, and the product “PaxView-TB/NTM”, which is designed to detect tuberculosis infection and non-tuberculous acid-fast bacteria, acquired the CE-IVD marking.
Besides, PaxGenBio completed the development of the STD examination product capable of performing contemporaneous diagnoses of chlamydia, gonorrhea, and syphilis, and the product capable of performing contemporaneous genotypic diagnosis of herpes virus.
One of the advantages of the products developed by PaxGenBio is the supply price, which is more than 50% cheaper compared to the products from other competitors.
President Park said, “... Our technology uses universal PCR devices that make our product essential for newly industrializing countries as well as underdeveloped countries.”, “... We are currently at the stage of securing sales bases in the South-East Asian countries, Eastern European countries, and African countries.”
President Park also stated, “... International Organizations such as UNICEF or WHO make significant efforts to conquer diseases prevalent in developing countries facing harsh environment. One of them is tuberculosis. The product of PaxGenBio, which is capable of diagnosing tuberculosis, is now participating in the KOICA (Korea International Cooperation Agency) CTS (Creative Technology Solution) Program on recognition of the product as the most appropriate product in satisfying sites in countries, which require quick identification of diagnostic results at a cheaper price.














![[BioS 레터]무균주사제 공급망 변화와 CDMO 대응](https://img.etoday.co.kr/crop/77/77/2262816.jpg)



