탐방
DCGen, “Analysis of RNA Expressions with NGS - a new prognostic diagnosis of breast cancer”
by Joungmin Cho
Development of ‘OncoFREE’, which applies its unique analysis algorithm to the analysis of the quantity of RNA expressions of 179 genes: “Predict the recurrence risk of Asian patients aged less than 50

▲Prof. Han Wonshik (Co-founder of DCGen)
DCGen was jointly founded in 2017 by Han Wonshik, Head of the Breast Cancer Center of Seoul National University Cancer Hospital, Prof. Shin Hee-Chul of the Surgery Department of Seoul National University Bundang Hospital, and Prof. Lee Han-Byoel of the Surgery Department of Seoul National University Hospital. It is developing diagnostics to predict the prognosis of breast cancer through NGS-based genome analysis.
DCGen has developed OncoFREE, a multiple-gene test based on Next Generation Sequencing (NGS), instead of the Polymerase Chain Reaction (PCR) used by the existing prognostic diagnostics. Prof. Han says, “OncoFREE® can analyze the quantity of RNA expression of 179 genes through its self-developed algorithm, and quantify the risk of distant metastasis into scores.”
In addition to OncoFREE, DCGen is developing ‘HOPE®’, a diagnostic product to analyze the risk of hereditary cancer, and ‘OncoPredict’, a product to analyze gene mutations in cancer somatic cells specific to breast cancer.
◇ NGS-based prediction of the prognosis of breast cancer through the analysis of the RNA expressions of 179 genes using its unique algorithm: “A high accuracy rate is shown among patients aged less than 50 years old”
OncoFREE is the product developed by DCGen that analyzes the quantity of RNA expression of 179 genes in tumor tissues of breast cancer patients to predict the future risk of distant metastasis by quantifying the results, with the aim of helping set up an appropriate treatment plan. Based on the cutoff prognostic score of 20 points, patients are divided into a low-risk group and a high-risk group. The targets of this test are hormone receptor-positive and HER2-negative breast cancer patients who account for about 70 % of all breast cancer patients.
Prof. Han says, “An anti-cancer treatment for a breast cancer patient is determined based on the size of tumors and the patient’s medical history after surgery. Doctors may have different opinions, and there are no grounds for accurate judgement. Thanks to the prognostic diagnosis using gene tests, we are better able to select the right patients than before.”
The differentiated feature of OncoFREE is that it is based on NGS. Oncotype DX developed by the U.S. Genomic Health uses the real-time PCR PCR(qPCR), while MammaPrint developed by Agendia, The Netherlands, uses a microarray method. Prof. Han says, “The PCR-based Oncotype DX can analyze 21 types of genes, while MammaPrint can analyze 70 types of genes. As OncoFREE can analyze more genes simultaneously than these products, it can produce more accurate results.” In the development process, DCGen developed and applied its unique analysis algorithm called the ‘Conserved Exon Method’ (CE method).
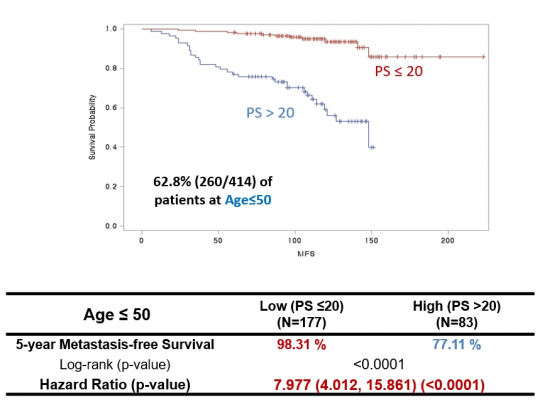
Prof. Han says, “After conducting prognostic diagnosis based on the likelihood of distant metastasis, we found the AUC produced far more accurate results than Oncotype DX.” The high-risk group classified by OncoFREE was found to have a higher recurrence hazard ratio (HR) by 6.6 times that of all patients, or by 7.8 times that of patients aged less than 50 years old. These numbers are far higher than the 2–3 times when patients are classified into high-risk or low-risk groups by their general physiological characteristics.
Prof. Han says, “Another advantage of OncoFREE is a higher prediction accuracy among patients aged less than 50 years.” As Oncotype DX was developed in reflection of the occurrence characteristics of Western women, it was mainly for women with menopause. When it is used for women aged less than 50 years, its accuracy and sensitivity tend to be decreased.
He says, “In the case of Asian patients, women in their 40s to 50s show a high prevalence of breast cancer. If prognostic prediction is done with overseas diagnostic products, accuracy can be reduced. OncoFREE developed by DCGen is found to show a high prediction accuracy in the analysis of samples collected from 260 patients aged less than 50 years.”
◇ Development of ‘Hope’ for the diagnosis of hereditary cancer risk, and ‘OncoPredict’ to provide the foundation for customized treatment; the company plans to expand the market segment of its diagnostic product from breast cancer to colon cancer, lung cancer, and so forth.
In addition to OncoFREE, DCGen is currently developing ‘Hope’, a diagnostic product for hereditary cancer, and ‘OncoPredict’ for the analysis of somatic cell gene mutation.
Hope is a diagnostic product that analyzes specific gene mutations with a high risk of cancer occurrence, and clinical data related to these gene mutations at the same time, in order to check the risk of hereditary cancer, and to provide a comprehensive treatment. Prof. Han says, “Hope analyzes multiple gene mutations by targeting a risk group with family history. It is quite difficult to determine whether mutations are actually related to a certain disease or not, rather than simple determination of the presence of mutations. However, DCGen has accumulated a great stock of clinical interpretation data and knowhow, based on about 60,000 cases of gene analysis results.”
Meanwhile, OncoPredict analyzes gene mutations in cancer somatic cells with NGS to explore and identify effective target treatments. In today’s medical field, gene analysis panels are mainly pan-cancer targeting products, but DCGen developed a diagnostic product that is specialized for breast cancer. A total of 121 genes to be targeted by substances were selected among genes with changes specific to breast cancer, and the test on actual patients was completed. The company says, “The simultaneous provision of OncoFREE and OncoPredict services is expected to be able to provide treatments that are customized to patients.”
Prof. Han says, “DCGen is the first company ever to develop an NGS-based diagnostic product that is applicable to actual patients beyond simple data production. We founded the company to provide better treatment to patients and to improve their quality of life, and will continue to do our best to develop products that can be helpful to patients.”






![[인사]한미그룹 2026년 정기 임원인사](https://img.etoday.co.kr/crop/74/74/2071974.jpg)







![[인사]한미그룹 2026년 정기 임원인사](https://img.etoday.co.kr/crop/77/77/2071974.jpg)



