기업
‘Fibrosis-Specialized’ Nextgene Biosciences’ Strategy for NASH, a New Target?
by Yoonseok Suh
Drug candidate ‘NXC736’ for NASH, a S1PR1/S1PR4 antagonist, submitting phase 1 IND this year. preclinical study of drug candidate ‘NCL600’ for radiation fibrosis of lung in progress

Nextgene Biosciences was founded in 2018 by CEO Bong-yong Lee, a former vice president of Daewoong Pharmaceutical. CEO Lee explained why he had started a new business. “I wanted to properly develop new drugs for intractable disease that can provide new life to patients. This is my lifelong mission.” CEO Lee said, “We want to develop drugs necessary for the next generation, not drugs available on the market right now.
While serving as director at Daewoong Pharmaceutical, Yuhan Corp., and SK Chemicals, CEO Lee has experience in technology transfer for several drugs. Currently, researchers who worked with him at Yuhan Corp., SK Chemicals, and Daewoong Pharmaceutical in the past have joined Nextgene Biosciences. They are challenging the development of new drugs for treating fibrosis.
While established, Nextgene attracted 1.5 billion won for Pre-Series A from Korean investment partners, etc. and 6 billion won for Series A in July 2019. About one year after that, in July of last year, it also attracted 20 billion won for Series B, raising about 30 billion won in total so far.
Nextgene targets fibrosis disease. The goal is to target indications with high unmet medical needs and achieve rapid optimization and early license-out. Accordingly, it is developing drugs by targeting diseases which one drug can be precisely applied to, instead of targeting multiple indications.
CEO Lee said, “For efficient and effective discovery of new drugs, we are focusing on exploratory research with an artificial intelligence (AI)-based drug discovery team, a medicinal chemistry team improving druglikeness, and a bio-evaluation research team t evaluating efficacy.” He emphasized that it was why they could obtain a large number of candidate substances while setting the laboratory at the same time for about a year after establishment.
He continued, “Through the open innovation strategy, we are reviewing feasibility and funding research after analyzing papers published by research teams in schools or hospitals that develop candidate substances we are targeting. Afterwards, to develop a variety of pipelines, we focus on the druggability part that Nextgene can do well.”
Nextgene Biosciences is currently developing pipelines for systemic fibrosis such as nonalcoholic steatohepatitis (NASH), idiopathic pulmonary fibrosis (IPF), and renal fibrosis, ophthalmological fibrosis such as wet/ dry maculopathy and diabetic retinopathy, radiation fibrosis of lung, and rare fibrosis such as rejection reaction after organ transplantation. Biospectator met Nextgene Biosciences to hear about the pipeline under development.
◆ S1PR1/S1PR4 functional antagonist ‘NXC736’ “Improving inflammation and fibrosis in mice and verifying safety”
Nextgene's lead candidate is NXC736, a drug candidate for nonalcoholic steatohepatitis (NASH). NXC736 is a functional antagonist for S1PR1 and S1PR4. Its action mechanism is to prevent fibrosis by inhibiting activities of NLRP3 inflammasome and pro-IL-1β. A functional antagonist means that acting as an agonist for a receptor, it functionally acts as an antagonist through downstream signaling process.
CEO Lee said, “NXC736 showed an effect of improving inflammation and fibrosis in mouse models of lung fibrosis and NASH.
Nextgene's NXC736 has been developed by Asan Medical Center and Seoul National University, and the technology has been transferred. Finding that myriocin had an effect of preventing NASH in an animal model, the research team of Asan Medical Center synthesized analogues of myriocin, and set out to develop new drug substances. The research team confirmed that NXC736 among several compounds responded specifically to S1PR1/4.
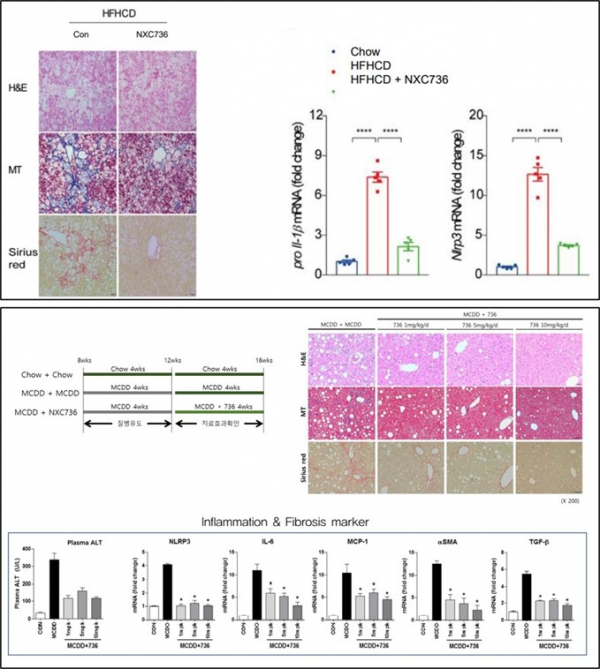
Nextgene confirmed anti-inflammatory and anti-fibrotic effects of NXC736 in a mouse model of diet-induced NASH. First, the expression of S1PR4 was increased in the NASH mouse model treated with high-fat/ high-cholesterol (HFHCD) diet. In addition, the expression levels of markers for inflammatory response and fibrosis (TNF-α, TGF-β, and MCP-1) and inflammasome marker such as NLRP3 and pro-IL-1β were increased in the HFHCD diet NASH mouse model. In contrast, they were significantly decreased in S1PR4 mutant mice (S1PR4-/+).
Then, if NXC736 is administered in a NASH mouse, will the result be similar to that of NXC736 administration in S1PR4 mutant (S1PR4-/+) mice? Nextgene confirmed that NLRP3 and pro-IL-1β were decreased upon NXC736 administration in an HFHCD-induced NASH mouse model. In another NASH mouse model on a methionine-choline-deficient diet (MCDD) for 4 weeks, following NXC736 administration for 4 weeks, the anti-fibrotic effect was confirmed, and the levels of anti-inflammatory biomarkers were significantly reduced.
Symptoms of radiation-induced pneumonia and pulmonary fibrosis were also relieved. Nextgene irradiated mice at 75 Gray (Gy) and administered NXC736 5 times a week. In general, pulmonary fibrosis occurs at 6 weeks after irradiation. As a result of analyzing various pulmonary fibrosis markers, the research team found that IC (inspiratory capacity) indicating the total volume of lung, Crs (compliance of the respiratory system) indicating lung compliance, Ers (elastance of the respiratory system) indicating lung resistance, H (tissue elastance) indicating lung tissue resistance, and Cst (Quasi-static compliance) indicating lung compliance were significantly improved.
NASH is a chronic inflammatory disease caused by fat accumulation in the liver for reasons other than alcohol consumption. NASH may worsen liver function damage to gradually develop into cirrhosis or liver cancer. So far, there is no treatment for NASH approved by the U.S. Food and Drug Administration (FDA).
Last year, obeticholic acid (OCA) of Intercept Pharmaceuticals, a leading group in the development of NASH treatments, failed to obtain approval from the U.S. FDA. Citing as the reason that the clinical benefit was not sufficient compared to the risk, the FDA requested additional efficacy and safety data from Intercept. OCA was shown to improve liver fibrosis in NASH patients in phase 3 clinical studies. However, more than half of the treated patients developed pruritus such as rash, and about 15% of them stopped taking the drug.
◆ ‘NXC518’ improves lung fibrosis indices in an irradiation-induced lung fibrosis model. “Starts preclinical study with an improved drug”
CEO Lee said, “Two-thirds of lung cancer patients receive radiation therapy, and about 75% of them develop radiation pulmonary fibrosis and radiation pneumonia. Although steroids and antibiotics are currently used as treatments, the long-term use of them are difficult due to toxicity and side effects. Therefore, this is a field with high unmet medical demand for fundamental treatment.”
Why does irradiation cause lung fibrosis? It is because endothelial-to-mesenchymal transition (EndMT) that changes the endothelial cells of pulmonary blood vessels into mesenchymal cells induces pulmonary fibrosis. CEO Lee said, "Control of radiation-induced damage to vascular endothelial cells is a very important target for early control of pulmonary fibrosis."
Nextgene analyzed the effect of candidate substance NCL518 in a model of lung fibrosis induced by irradiation with 90 Gy of radiation. NCL518 was administered 1 hour before irradiation (pre-treatment). On 14 days after irradiation, the mice were dissected, and lung tissues were analyzed.
As a result, the trichrome-positive range indicating the degree of collagen deposition and the Ashcroft score indicating lung fibrosis were significantly reduced compared to those of the positive control group. Radiation-induced metastasis of vascular endothelial cells to mesenchymal cells was also inhibited. “This year, we are scheduled to conduct a preclinical study with a drug called NCL600 that has more improved effects than NCL518,” said CEO Lee.
Humanetics Pharmaceuticals of the United States is undergoing Phase 1b/2 clinical study (NCT02567799) of 'BIO300', an estrogen receptor (ER) agonist, since 2015 to see the effect of improving radiation therapy-related pneumonia and pulmonary fibrosis in patients with non-small cell lung carcinoma (NSCLC).





![[인사]셀트리온그룹 2026년 임원승진 인사](https://img.etoday.co.kr/crop/74/74/2274343.jpg)







![[인사]셀트리온그룹 2026년 임원승진 인사](https://img.etoday.co.kr/crop/77/77/2274343.jpg)


