기업
Bridge Biotherapeutics "BBT-877, US IND will be submitted by year end"
by Sungmin Kim
Bridge Biotherapeutics Presented Preclinical Study Results on BBT-877, an Autotaxin inhibitor at the IPF Summit 2018
Bridge Biotherapeutics, a clinical stage biotech company headquartered in Seongnam, South Korea, presented the results of the preclinical study on ‘BBT-877’, an investigational drug candidate for IPF(Idiopathic Pulmonary Fibrosis) treatment, at the IPF Summit 2018 held in San Francisco, California.
Bridge Biotherapeutics now plans to submit a US IND of BBT-877 by the end of this year.
BBT-877 was discovered by LegoChem Biosciences and licensed to Bridge Biotherapeutics for the worldwide exclusive right for further development. BBT-877 targets Autotaxin(ATX), which has emerged as a promising target for various diseases such as fibrosis and cancers.
GLPG1690, an ATX inhibitor being developed by Galapagos has been approved to proceed to phase 3 clinical trial bypassing phase 2b study in the U.S. after showing promising results of phase 2a performed with 23 IPF patients.
Bridge Biotherapeutics presented the results of BBT-877 preclinical study at the poster session of the IPF Summit 2018, which demonstrated strong efficacy in the bleomycin-induced mouse model. The study indicates that BBT-877 has effectively reduced lung fibrosis as presented by reduction of Ashcroft scores and the deposition of collagen(staining), compared to the other drugs. The biotech expects that the data implies a potential of BBT-877 to be the ‘best-in-class’ drug for IPF treatments.
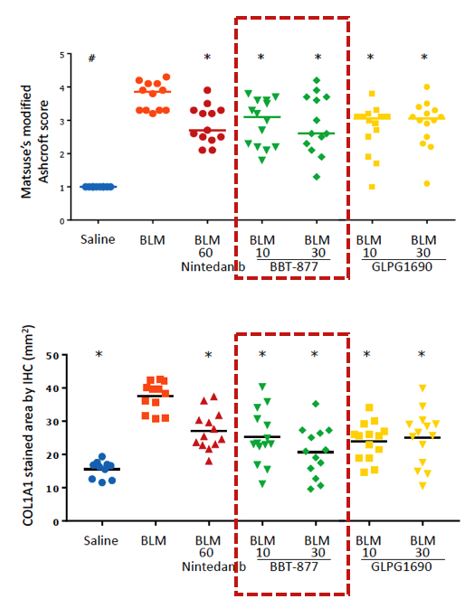
▲Figure 1. Key results from pre-clinical study on BBT-877 efficacy
“It is a great opportunity for Bridge Biotherapeutics to present the outstanding preclinical study results on BBT-877 at the IPF Summit 2018. Bridge Biotherapeutics aims to develop BBT-877 as the best-in-class drug for IPF as fast as possible to bring this investigational compound to patients as new treatment option.” said James Lee, CEO of Bridge Biotherapeutics.


















