기업
Anterogen, diabetic foot ulcer patch “encouraging efficacy in phase 2 trial”
by Sungmin Kim
Published in a foreign journal ‘diabetes’ … patient ratio of complete wound recovery (closure) and its time spent was reduced after 12 weeks of allogenic mesenchymal stem cell patch ‘ALLO-ASC-sheet’ …

The clinical results showing the possibility of efficacy of ‘ALLO-ASC-Sheet’, Anterogen’s mesenchymal stem cell patch for diabetic foot ulcers, were published in foreign journal. It is the result of phase 2 trial in Korea for subjects with diabetic foot ulcers that published in ‘Diabetes’ with the title of ‘Potential of allogenic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers’ on January 24th.
Anterogen is currently conducting a phase 3 trial of ‘ALLO-ASC-DFU’ (project name) for the treatment of diabetic foot ulcers in Korea (NCT03370874), phase 2 in the U.S.(NCT03754465), and both will be over in this year.
Also, designation as a ‘Sakigake’ about conditional item approval of another indication for ‘ALLO-ASC-DEB’ as a treatment for rare dermal condition, dystrophic epidermolysis bullosa, will be determined in this March from Japanese government. Sakigake is an expedited review system, and the designated items can get the approvals within 6 months and its prices about 10 ~20% premium. Also, Anterogen got approved for phase 1 clinical trial in the U.S. for the same indication.
ALLO-ASC-Sheet is a hydrogel patch containing allogenic mesenchymal stem cells derived from adipose tissue. This product has its unique distinction that it can be frozen for one year and be readily made for the patient. Conventional mesenchymal stem cell therapy drugs have difficulties in commercialization, because it is necessary for doctors to inject them into patients through surgery. To solve these limitations, Anterogen has made it a mass-produced and exportable patch.
Diabetic foot ulcers are symptoms of foot ulcers or sores. Currently, there is no cure for it yet. Anterogen’s patch contains various growth factors such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) that is secreted by the mesenchymal stem cells, extracellular matrix protein, and anti-inflammatory factor, and it is believed that the wound is recovered and the tissue regeneration is accelerated by these factors.
This phase 2 trial was conducted on 59 patients with diabetic foot ulcers. Among them, ALLO-ASC-Sheet was prescribed to 30 patients, and topical polyurethane film to 29 patients as a control (NCT02619877). ALLO-ASC-Sheet and topical agent are applied once a week for up to 12 weeks to the affected area. The clinical endpoint is the percent ratio of patient with the complete wound closure after 8 and 12 weeks of attaching the patch.
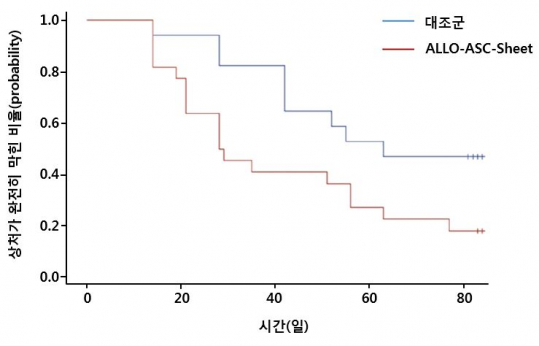
As a result, the lesions were completely sealed in 73% of the ALLO-ASC-Sheet treated group and 47% of the control group at 12 weeks. The efficacy of patches was better in the group with severe severity. The subgroup analysis from Wagner grade 1 (superficial diabetic ulcer) with a good lesion condition showed 85.7% (12/14) in ALLO-ASC-Sheet treatment group and 72.7% (8/11) in control. On the other hand, Wagner grade 2 patients in treatment difficulties with severe symptoms showed a greater difference in treatment efficacy from total wound closure rate of 75.0% (6/8 patients) in ALLO-ASC-Sheet treated group and 16.7% (1/6 patients) in control group.
The median time to complete wound closure (Kaplan-Meier median time) as another clinical endpoint was 63.0 days in the control group and 28.5 days in the ALLO-ASC-Sheet group (p=0.033). The rate of actual wound size reduction was accelerated with attaching ALLO-ASC-Sheet. The rate at which the wound was reduced was statistically significant at the first week (49.6% vs 23.0%, p=0.007) and at 9th week after prescribed. There were no side effects associated with attaching ALLO-ASC-Sheet.
Anterogen will present results of this phase 2 clinical trial at the SAWC/WHS conference in Texas on this May. At the SAWC/WHS conference, more than 100 institutions with 2000 doctors, scientists, and industry experts participating in every year announce the wound treatment for variety of diseases.





![[인사]유한양행, 2026년 1월 임원 인사](https://img.etoday.co.kr/crop/74/74/2044634.jpg)

![[인사]일동제약그룹, 임원인사 발령](https://img.etoday.co.kr/crop/74/74/2275874.jpg)




![[인사]유한양행, 2026년 1월 임원 인사](https://img.etoday.co.kr/crop/77/77/2044634.jpg)




