탐방
Exostemtech, 'Adipose-derived Exosome' "Clinical Trial for OA in Next Year"
by Yoonseok Suh
Intra-articular injection 'CARTISOME', a candidate drug for degenerative osteoarthritis (OA), is expected to enter the phase 1 clinical trial in the second half of next year..Phase 1b/2a clinical tria

Exostemtech is conducting a clinical trial of exosome-based therapeutics for the first time in Korea. Exostemtech plans to submit a phase 1 clinical trial protocol(IND) for degenerative osteoarthritis (OA) using exosomes from adipose-derived stem cells early next year and start the clinical trial in the second half of next year. As the leading group, Codiak Biosciences in the US, started phase 1 clinical trials last year, Exostemtech can be said to be at a global level in terms of development speed.
Exostemtech was founded in 2016 by Professor Yong-woo Cho of Hanyang University ERICA campus and attracted 4 billion won in Series A investment in 2019. Following 2.5 billion won in a bridge round investment last year, they raised 10 billion won of series B round funding in October of this year. They plan to apply for technology evaluation for listing on the KOSDAQ in the second half of next year. In addition, Exostemtech transferred exosome-based cosmeceutical technology to ExoCoBio in Korea in 2017 and is receiving running royalties every year.
Yongwoo Cho, CEO of Exostemtech, said, “We plan to conduct phase 1 clinical trial of CARTISOME, the first exosome-based osteoarthritis treatment in Korea, next year. In addition, we have received approval for phase 1b/2a clinical trial of the drug, which inhibits the exosomes secretion from cancer, in combination therapy with Keytruda, for solid cancer." Also, Exostemtech is developing 'HEPATOSOME', a candidate treating for hepatic fibrosis, and 'OSTEOSOME', a candidate for treating osteoporosis. Biospectator looked into Exostemtech's pipeline, production technology, and clinical development status based on exosomes.
◆ ‘CARTISOME’, a Candidate for Treatment of Degenerative Osteoarthritis(OA)…“Entering Phase 1 Clinical Trial in Next Year”
Degenerative osteoarthritis(OA) is a disease in which knee cartilage wears down due to aging, obesity, genetic factors, and injuries. Exostemtech expects that CARTISOME will treat degenerative OA with three mechanisms: anti-inflammatory effects, cell differentiation and migration, and homeostasis recovery.
First, CARTISOME suppresses the activation of M1 macrophages, which are inflammatory immune cells, and reduces the expression level of the MMP family, which are cartilage degrading enzymes . At the same time, it induces differentiation of M1 macrophages into M2 macrophages, which are anti-inflammatory immune cells. M2 macrophages decrease the inflammatory response to relieve pain and induce tissue regeneration. CARTISOME proliferates chondrocytes and allows the cells to migrate to the damaged area. Finally, it promotes the synthesis of extracellular matrix(ECM) protein secreted by chondrocytes.
CEO Cho said, “CARTISOME can be administered to patients with early to moderate osteoarthritis, and it has the distinction of being administered once into the joint cavity without surgery and able to administer multiple times. The conventional drugs use a method of filling the drug by drilling a small hole in the cartilage defect for moderate to severe patients, and it is difficult to administer multiple doses because of the immune response."
Exostemtech confirmed the cartilage regeneration effect in an arthritis animal model administered with CARTISOME(doi: 10.1080/20013078.2020.1735249). First, the research team confirmed that the mRNA and protein expression of the cartilage degrading enzymes MMP-1, MMP-3, MMP-3, and ADAMTS-5 increased when IL-β1 was treated to induce an inflammatory response in osteoarthritis chondrocytes. The mRNA and protein expression of increased cartilage degrading enzymes were significantly reduced to normal levels when CARTISOME, which is adipose-derived stem cells EV(hASC-EVs).
Additionally, the research team confirmed the cartilage regeneration effect by administering CARTISOME to a monosodium iodoacetate (MIA)-induced osteoarthritis(OA) rat model or surgical destabilization of the medial meniscus(DMM) model. The research team injected MIA into the rat and administered PBS (phosphate buffer saline), CARTISOME, and hyaluronic acid(HA) once a week for 3 weeks from 1 week later. This model was defined as a subacute model before the OA progressed sufficiently. The Mankin score, analyzed by cartilage structure and cellularity, was significantly decreased in the subacute model administered with CARTISOME compared to the PBS and HA administration groups, confirming the cartilage regeneration effect.
Also, in the chronic model in which MIA was injected into rats and CARTISOME was administered 3 times every 2 weeks from 2 weeks later, the Mankin score was significantly decreased compared to that of the PBS-administered group but was at a similar level to that of the HA-administered group In the cartilage tissues of the subacute and chronic models administered with CARTISOME, the number of IL-b1 cells that induce matrix metalloproteinases(IL-1b positive cells) was approximately 10 (vs about 40) and 30 (vs about 80), respectively, which was reduced compared to the PBS-administered group.
As a result of administering CARTISOME 6 times a week to the surgical DMM model, the OARSI index was significantly improved, and the cartilage tissue was restored to the normal tissue level. In addition, the expression of MMP-13, a cartilage degrading enzyme, was also significantly reduced in cartilage tissue. The OARSI index evaluates the degree of osteoarthritis on a scale of 0 to 6, with higher numbers indicating more severe.

◆ ‘EST-SFX-T’ that inhibits tumor-secreted exosome…”Phase 1b/2a clinical trial in combination with Keytruda in progress”
CEO Cho said, “Most exosomes represent the function of parent cells that secrete them. Tumor-derived exosomes have functions related to cancer metastasis and growth, so we are conducting phase 1b/2a clinical trials of a drug, which inhibits tumor-derived exosomes, in combination therapy with the PD-1 antibody Keytruda for solid cancer."
Exostemtech approached this by suppressing exosome secretion from cancer cells to enhance the anticancer effect of immunotherapy, based on the research results that exosome secreted from tumor cells lowered the effect of immuno-oncology drugs such as Keytruda.
Exostemtech used sulfisoxazole(SFX), an antibiotic approved by the US Food and Drug Administration (FDA), as a drug repositioning method. SFX is known to inhibit exosome production and secretion from tumor cells. In particular, it blocks exosome secretion by interfering with endothelin receptor A(ETA) overexpressed on the surface of tumor cells (doi.org/10.1038/s41467-019-09387-4).
Exostemtech is currently conducting a phase 1b/2a clinical trial for the combination of ‘EST-SFX-T+Keytruda’ in 26 patients with advanced solid cancer together with Professor Jinhyeong Kang of The Catholic University of Korea Seoul St. Mary’s Hospital. CEO Cho said, “First, tumor-derived exosome reduction and safety after drug administration are confirmed, and then we will increase the number of patients for indications such as lung cancer and proceed with combined clinical trials. It is a strategy to conduct initial clinical trials in Korea and conduct global clinical trials from phase 2b, or transfer technology.
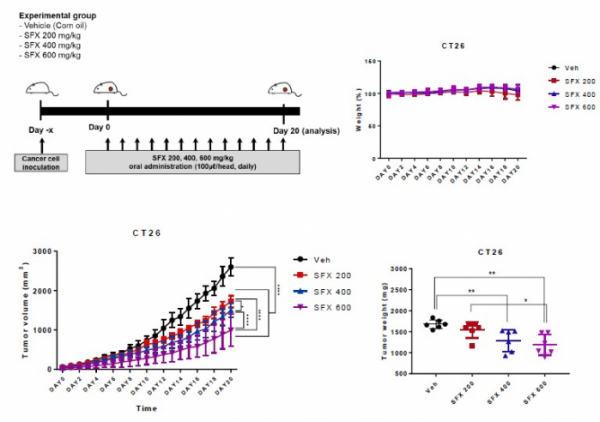
As a result of daily administration of SFX at 200, 400, and 600 mg/kg doses to the CT26 colon cancer mouse model, it was confirmed that the tumor size decreased in a dose-dependent manner. In addition, in the CT26 colon cancer mouse model in which SFX 200mg/kg and PD-1 antibody were administered together, the tumor weight was significantly reduced compared to the SFX or PD-1 antibody alone group. The PD-L1 concentration in the tumor-secreted exosome increased compared to the control group when the PD-1 antibody was administered alone and decreased to a normal level when the SFX+PD-1 antibody was administered in combination.
In addition, they administered SFX, PD-1 antibody, and SFX+PD-1 antibody to a colon cancer mouse model and analyzed the tumor size on the 21st day. As a result, the tumor size was about 1500㎣ in the control group, and it was confirmed that the tumor size was reduced to about 1000㎣, 750㎣ for the SFX and PD-1 antibody-administered group, respectively, and about 250㎣ for the SFX+PD-1 antibody-combined administered group.
Why is Exostemtech conducting the clinical trial for the combination of SFX and Keytruda? First, PD-L1 protein, expressed by exosome secreted from tumor cells, inhibited CD8 T cell activity and was highly expressed in non-responders of Keytruda, which was associated with the prognosis of patients treated with cancer immunotherapy(doi.org/10.1038/s41586-018-0392-8). Also, when PD-L1 expressed on the exosome surface was inhibited, the anticancer effect was enhanced and T cell activity was increased(doi.org/10.1016/j.cell.2019.02.016).
According to the specific study results, first, PD-L1, expressed in exosomes secreted from melanoma, suppressed CD8 T cell activity and cytokine production, thereby lowering the immune response. Melanoma-derived exosomes were injected into the actual mouse model and analyzed 24 days later. As a result, the tumor size increased to about 2500㎣. The tumor size of the control group was about 500㎣, and the tumor size decreased to about 1000㎣ when exosomes were injected with the PD-L1 antibody.
Patients with stage 3 or 4 melanoma who did not respond to PD-1 antibody, Keytruda(non-responder, NR) showed a significantly higher expression of exosome-derived PD-L1 in the blood (p=0.0001). In addition, the overall response rate(ORR) to Keytruda in melanoma patients was 77% (14/18 patients) in patients with exosome PD-L1 concentrations lower than 1.03 ng/mL and 26.9% (7/26 patients) in those with exosome PD-L1 concentrations higher than 1.03 ng/mL. The results of the study suggest that the effect of Keytruda treatment may vary depending on the concentration of exosome PD-L1 in the blood(doi.org/10.1038/s41586-018-0392-8).
Let's look at the results of another study in which exosome inhibition of PD-L1 expression enhances the anticancer effect. The UCSF research team, led by Professor Robert Belloch, analyzed the function of exosome PD-L1 using TRAMP-C2 WT, a mouse model of prostate cancer.
In the mouse model of prostate cancer, after 50 days, the tumor size increased from 0㎣ to 1000㎣, and the survival rate decreased to about 10%. On the other hand, tumors did not grow and the survival rate was about 75% or more up to 100 days in the Rab27a mutant (TRAMP2 Rab27a-/-) related to exosome biogenesis, the nSMase2 mutant related to PD-L1 expression and secretion(TRAMP2 nSMase2-/-), and the mouse prostate cancer model in which the PD-L1 gene was removed by CRISPR.
In mouse models injected with colorectal cancer cells, MC38, Rab27a mutant MC38, and PD-L1 mutant MC38 cells, tumor growth was suppressed and survival rate increased. Specifically, the MC38 mouse model showed a tumor size of about 500㎣ on the 15th day, and the Rab27a mutant MC38 and PD-L1 mutant MC38 mouse models showed a tumor size of 100-200 mm 3 on the 23rd day. The survival period was about 20 days in the MC38 mouse model, whereas it was more than 30 days in the Rab27a mutant MC38 and PD-L1 mutant MC38 mouse models. Additionally, the research team confirmed the result of increased immune cells such as CD8 T cells and GzmB cells in the lymph node of Rab27a mutant and PD-L1 mutant mice(doi.org/10.1016/j.cell.2019.02.016).
◆ Development of exosome-based therapeutics "What is the global competition?"
At the global level, the development of exosome-based therapeutics is from a preclinical to the early stage of phase 1/2 clinical trials. Currently, Codiak Biosciences, known as the leading group, is carrying out phase 1/2 clinical trials and most other companies are competing in the preclinical phase.
Codiak Biosciences developed the EngEX platform, which increased stability and activity by expressing high levels of the exosome-specific protein PTGFRN. Codiak Biosciences is developing drugs that deliver ‘ExoSTING’, which carry STING agonists that stimulate immune responses by applying EngEX technology, ‘ExoIL-12’, in which IL-12 was directly bound to PTGFRN, and STAT6 antisense oligonucleotide(ASO).
Another competitor, Evox Therapeutics, has a platform technology that targets specific tissues and delivers drugs. Specifically, Evox Therapeutics' DeliverEX platform is a technology to express a specific-tissue-targeting protein on the exosome membrane and to load and deliver mRNA, RNAi, ASO, etc. used as drugs in the exosome.














![[인사]일동제약그룹, 임원 승진 인사](https://img.etoday.co.kr/crop/77/77/1644212.jpg)



![[인사]일동제약그룹, 임원 승진 인사](https://img.etoday.co.kr/crop/74/74/1644212.jpg)
