오피니언
Top Trends for 2018 Oncology Research & Development
Experienced Expert for Oncology in Global Pharmaceutical Company, Jeonghoon Han Ph.D
3 Major Trends in Oncology: Advances in Immuno-Oncology, Emerging Antibody Drug Conjugate, Precision Medicine
During the past decade, there has been a paradigm shift in the treatment of cancer, driven by advances in immuno-oncology, antibody drug conjugate and precision medicine. Since 2011, more than 80 novel therapies have been approved globally for treatment of cancer. In addition to the recent wave of newly launched therapies, the late-stage oncology pipeline is robust with over 600 molecules in development. The focus on oncology will remain high over the next decade driven by the ongoing research and development and remaining unmet need.
Advances in Immuno-Oncology

▲Table 1: Development status of PD-1/PD-L1 agents in oncology
Immuno-oncology agents have significantly changed the treatment paradigm in many of the cancer types. In particular, PD-1 and PD-L1 inhibitors have witnessed a rapid uptake based on their remarkable clinical profile and approval for multiple cancers. Table 1 shows the current status of PD-1 (Opdivo and Keytruda) and PD-L1 (Tecentriq, Imfinzi and Bavencio) that have been actively developed and approved in many cancer types, especially in the cancer types that have high unmet need (Current medicines have not shown strong efficacy). The five main PD-1/PD-L1 agents have been developed in most of cancer types as monotherapy or in combination with targeted therapy, immune-oncology agents and chemotherapy. Some of PD-1/PD-L1 agents have already been approved for melanoma, non small cell lung cancer, renal cell cancer, hodgkin’s lymphoma, head and neck cancer, hepatocellular cancer, gastric cancer and colorectal cancer that are in high unmet need.
Even for bladder cancer that drew less interest from the pharmaceutical industry due to low incidence, interestingly, all the PD-1/PD-L1 inhibitors (Opdivo, Keytruda, Tecentriq, Imfinzi and Bavencio) have been approved for use in 2nd line bladder cancer.
Similar for small cell lung cancer, Brain cancer, Esophageal cancer, Pancreatic cancer and Ovarian cancer, where few targeted therapies are available and therefore high unmet medical needs, it is anticipated that immuno-oncology agents may achieve a significant progress in these desperate cancer types.
With the expected late launch in these already crowded immune-oncology markets, Bavencio, the fifth PD-1/PD-L1 agent, has taken quite different approaches. Only Bavencio has already obtained the indication of merkel cell carcinoma that is very niche market. In addition, Bavencio has the indication as a maintenance therapy following platinum-containing chemotherapy in locally advanced or metastatic urothelial carcinoma.
The most interesting cancer type with respect to the development of PD-1/PD-L1 inhibitors is non small cell lung cancer (NSCLC). The advanced or metastatic NSCLC has quite a significant market size since detection of NSCLC is often in late stage. Even though it is mostly treated in locally advanced or regional stage of the disease, most of cases recur or relapse. Thus, the final goal of NSCLC drug development is to get into the position of 1st line treatment for advanced NSCLC.
The key landmark data regarding Opdivo and Keytruda have been reported in 2016 and 2017. There have not been new statistically significant overall survival (OS) data in first line setting for more than a decade except in ALK+ or EGFR+ NSCLC patient types. In June 2016, Merck’s KEYNOTE-24 study showed a PFS and OS benefit for Keytruda in first-line NSCLC vs. chemotherapy in patients with PD-L1 ≥50%. KEYNOTE-24 study was selective of PD-L1 ≥50%. However, in August 2016, the CheckMate 026 of Opdivo failed in first-line patients with PD-L1 ≥5%. Furthermore, Keytruda plus platinum/pemetrexed therapy (Randomized Phase II, KEYNOTE-021) showed a PFS and OS benefit in nonsquamous, first-line patients with all PD-L1 expression levels and have been approved in May 2017. Thus, Keytruda monotherapy is standard-of-care, first-line therapy for patients with ≥50% PD-L1 expression and Keytruda plus platinum/pemetrexed therapy is an option for untreated patients with advanced non-squamous NSCLC with PD-L1 expression <50%.
In terms of market differentiation among PD-1/PD-L1 agents, six strategies are currently implemented in global pharmaceutical or biotech companies.
• Firstly, to achieve superior data in key patient types such as in first line of advanced NSCLC patients.
• Second, to look for opportunity in niche market such as Bavencio in merkel cell carcinoma.
• Third, to combine with other agents that have a potential for improved efficacy, safety and cost.
• Fourth, unique patient settings that offer new treatment options in areas of less competition and unmet need such as Bavencio in a maintenance therapy following platinum-containing chemotherapy in locally advanced or metastatic urothelial carcinoma.
• Fifth, biomarker strategies. Keytruda focused on PD-L1-positive NSCLC study first, then expanded to all PD-L1 levels in combination with chemotherapy. Next, is to find more selective and predictive markers beyond PD-L1, such as mutation tumor burden, gene signatures, PD-L2 and inflammatory biomarkers in order to maximize cost effectiveness.
• Sixth, to utilize PD-1/PD-L1 inhibiters in earlier setting (adjuvant or neo-adjuvant) in the course of tumor progression to achieve more cures.
The future R&D direction of checkpoint modulators is to identify and develop new checkpoint modulators such as TIGIT, LAG-3, OX-40, GITR and I21R. The next level of checkpoint modulator development is the conjugate of PD-1/PD-L1 agent with new checkpoint modulators to enhance activity of intra-tumoral T cells.
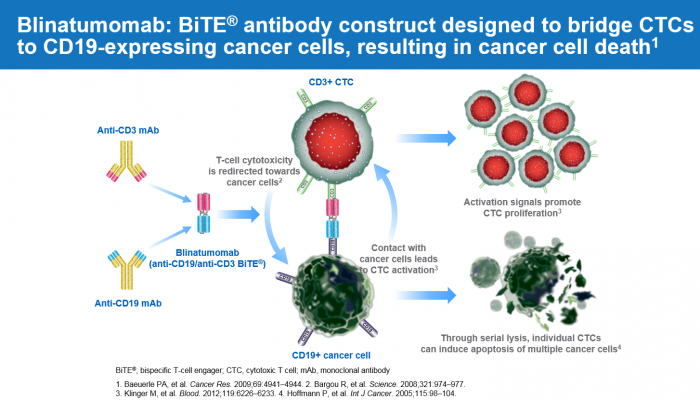
▲Figure-1. Blinatumomab: BiTE® antibody construct designed to bridge CTCs to CD19-expressing cancer cells, resulting in cancer cell death
Other important immune-oncology classes are BiTE and CAR-T technologies that are gaining significant interests in acute lymphoblastic leukemia (ALL). Blincyto has been developed based on BiTE technology (Please refer to the figure-1) and obtained FDA approval in ALL in 2014. Blincyto showed approximately 40% of complete response rate in relapsed or refractory ALL patients. Surprisingly, CAR-T showed more than 80% of complete response rate in relapsed or refractory ALL patients. However, CAR-T has its limitation of high cost, limited production facilities, long manufacturing time and risk of failure of product. Half life extension (HLE) forms of BiTE and Universe CAR-T and CAR-NK are being developed to overcome their limitations.
The main treatment goal in ALL is to achieve complete response (CR) in the first line treatment and then MRD (minimal residual disease) negativity. If MRD is positive, allogenic transplantation should be done. Thus, Blincyto or CAR-T as mono or in combination/sequential with chemotherapy studies have been conducted in first line ALL to access achievability of CR and MRD negativity.
Emerging Antibody Drug Conjugate
Over the past couple of decades, antibody drug conjugates (ADCs) have revolutionized the field of cancer chemotherapy. Unlike conventional treatments that damage healthy tissues upon dose escalation, ADCs utilize monoclonal antibodies (mAbs) that specifically bind tumour-associated target antigens and deliver a highly potent cytotoxic agent. The synergistic combination of mAbs conjugated to small-molecule chemotherapeutics, via a stable linker, has given rise to an extremely efficacious class of anti-cancer drugs with an already large and rapidly growing clinical pipeline.
The first ADC is Mylotarg (Gemtuzumab ozogamicin) that had the indication of acute myeloid leukemia from 2000 to 2010. It was withdrawn from market in June 2010 when a clinical trial showed the drug increased patient death and added no clinical benefit over conventional cancer therapies. However, it was approved again in Sep 2017 by the US FDA with new data, different dose and schedule.
The next ADC is Adcetris (Brentuximab vedotin) that achieved the indication for relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL) in 2011. In November 2017, it has expanded the indication to primary cutaneous anaplastic large cell lymphoma (pcALCL) and CD30-expressing mycosis fungoides (MF) patients who have received prior systemic therapy.
The third one is Kadcyla (T-DM1, ado-trastuzumab emtansine). In 2013, Kadcyla obtained the indication for the treatment of patients with HER2-positive, metastatic breast cancer who previously received trastuzumab and a taxane. It showed the OS benefit of 30.9 months as well as PFS that demonstrated the potential of ADC in terms of the OS efficacy with mild side effect.
The fourth is Besponsa (inotuzumab ozogamicin) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). 80% of complete response rate is impressive in this patient setting.
Adcetris has initiated the Ph III trial of CHECKMATE 812 in combination with nivolumab for relapsed/refractory Hodgkin lymphoma. Mirvetuximab soravtansine, Immunogen pipeline, has on-going Ph III trial in platinum-resistant ovarian cancer patients that are folate receptor alpha-positive with up to 3 prior regimens.
The success of future ADCs relies on improving target selection, increasing cytotoxin potency, developing innovative linkers and overcoming drug resistance. As more research is conducted to tackle these issues, ADCs are likely to become part of the future in targeted cancer therapeutics.
Precision Medicine
According to the National Cancer Institute ([NCI], 2017c), precision medicine is “a form of medicine that uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease. In cancer care, precision medicine uses specific information about a person’s tumor to help diagnose, plan treatment, monitor the effectiveness of treatment, or make a prognosis.” Unnecessary treatments also can be avoided if the individual’s profile does not match a particular therapy.
Cancer treatment has seen increased focus on precision medicine, leading to patient segmentation based on biomarker status. Major cancer types (lung, breast, colorectal (CRC), melanoma) have become increasingly segmented, with each segment now being recognized as having specific treatment options and outcomes. In many cases, cancer is no longer a single tumor type diagnosis but is defined by a combination of factors, including histology and biomarker status. Identification of newer biomarker niches, such as microsatellite instability (MSI) status in CRC, and BRAF (a gene that encodes B-raf) and PD-L1 (programmed cell death protein ligand 1) status in NSCLC, are likely to fragment the patient populations within these cancers further.

▲Figure-2. ESMO Guidelines Committee: Zurich Treatment Algorithm
Colorectal cancer (CRC) is a good example for precision medicine (Figure-2). Metastatic CRC (mCRC) patient segmentation is based on resectability, genetic marker (RAS, BRAF) and tumor location (Left vs. Right). mCRC patients should first be segmented based on tumor resectability, ability for cytoreduction (shrinkage of tumor) and disease control (control of tumor progression). Thereafter, molecular markers like RAS and BRAF mutational status should be defined. The 2017 new guideline has also accepted tumor location (Left vs. Right) as prognostic and predictive factors. Thus, treatment recommendation of ESMO 2017 guideline based on the tumor location differs and has translated into real clinical practice. Microsatellite Instability (MSI) High is also another significant biomarker in CRC patients. MSI High biomarker has been approved from FDA in 2017 as the first indication of biomarker, not by cancer type and histology.
Oncology continues to be an area of active interest with a robust pipeline of which 87% is targeted therapy; several of the targeted therapies in development have an associated biomarker. Targeted agents inhibit the growth and spread of cancer by interfering with specific molecular targets involved in cancer progression. Alongside personalized medicine with targeted agents, the approval of novel immunotherapy agents which provide substantial clinical benefit, have raised the hope of significantly improving cancer survival across a large number of tumor types.














