탐방
ImmunAbs, developing a novel C5 antibody for complement-associated autoimmunity
by Yoonseok Suh
Anti-C5 antibody ‘IM-101’ showed effective inhibition of C5 activity compared to existing drug in preclinical studies..expecting final results of the phase 1 trial in the U.S. at the end of this year

ImmunAbs has embarked to develop complement-associated autoimmune disease treatment using a next-generation complement 5 antibody that exhibits improved C5 inhibitory efficacy and safety.
Dr. DongJo Kim, CEO of ImmunAbs, stated, "Based on the preclinical results of inhibiting C5 activity, which is more effective than existing drugs with higher target selectivity, we are currently conducting a phase 1 clinical trial in the U.S. for the lead pipeline, IM-101. We have also scheduled biomarker analysis such as free C5, to assess effectiveness of the treatment. We believe that the end of this year, when the final results are expected, will be a significant inflection point”
Established in 2017, ImmunAbs is led by CEO Dr. DongJo Kim, formerly a senior manager of the global technology business unit at Celltrion, and Dr. Kisu Kim, head of R&D, who served as the leader of the antibody engineering team at the Mogam institute for biomedical, and team leader-level personnel who were in charge of the manufacturing process(CMC) at GC, and preclinical study, clinical study, QA/QC at Celltrion joined to conduct research and development. ImmunAbs attracted 11.6 billion won in Series A in 2021 and 13 billion won in Series B last year.
IM-101, the lead pipeline of ImmunAbs, is an antibody therapeutic that targets and inhibits C5 in the complement system, a key gateway molecule in autoimmune diseases. IM-101 targets and inhibits a different site from the existing approved drugs, AstraZeneca/Alexion's C5 antibody 'Soliris(eculizumab)' and 'Ultorimis(ravulizumab)'. ImmunAbs is currently analyzing the safety and tolerability of IM-101 in a phase 1 clinical trial in the US and expects to have final results by the end of this year.
Aprt from confirming the higher C5 inhibitory activity of IM-101 than the existing C5 antibodies in preclinical studies, ImmunAbs positively evaluated the fact that no special safety issues have emerged in the clinical trial conducted so far. ImmunAbs plans to develop IM-101 for indications for generalized myasthenia gravis(gMG) and neuromyelitis optica spectrum disorder(NMOSD) in the future.
‘IM-101’ as a Lead Pipeline C5 Antibody..Expecting final results of a phase 1 trial in the US at the end of this year
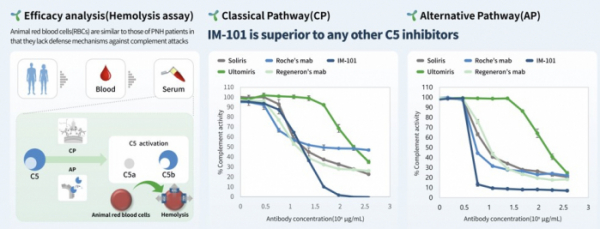
The complement system activated by foreign pathogens such as microorganisms, viruses, cancer cells, and antigens, plays a role in supporting the immune response through opsonization. Opsonization involves antibodies surrounding pathogens to facilitate their dissolution by phagocytes. The complement system consists of the classical pathway induced by antibodies , the alternative pathway activated independently of antibodies, and the lectin pathway activated by carbohydrates on foreign pathogen surfaces. The classical pathway begins with the C1 complex composed of C1q/r/s(C1qrs), followed by external substances through the C1 complex → C2, C4 activation → C3 activation → C5 activation. During complement activation, proteins such as C5b are produced to form a membrane attack complex(MAC), creating pores to eliminate cells. Activation of the complement system is associated with various autoimmune diseases such as paroxysmal nocturnal hemoglobinuria(PNH), atypical hemolytic uremic syndrome(aHUS), generalized myasthenia gravis(gMG), and neuromyelitis optica spectrum disorder(NMOSD). It is known that one or both classical and alternative pathways are related to the onset of these diseases. According to ImmunAbs’ study, the lead pipeline, IM-101, inhibited the C5 activity of the classical and alternative pathways more effectively than competing drugs. ImmunAbs analyzed C5 activity by treating IM-101 and rival drugs such as Soliris and Ultorimis in 0.7-375ug/mL doses under conditions similar to the physiological environment of the body(25% serum) using blood obtained from healthy people. As a result, the C5 activity of the classical pathway was inhibited by up to 60-70% in the Soliris-treated and Ultorimis-treated groups, while the IM-101-treated group inhibited it close to 100%. The C5 activity of the alternative pathway was inhibited by more than 90% in IM-101, whereas Soliris and Ultorimis showed 70-80% inhibition.
In fact, Soliris did not completely inhibit C5 activity in some PNH patients in the long run, requiring further treatment. As a result of a real-world study that followed patients treated with Soliris for an average of 5.59 years, 38%(29/76) of patients required blood transfusion, and at some point during follow-up, breakthrough symptoms, breakthrough hemolysis(breakthrough hemolysis) and transfusion dependence occurred in 63%, 43%, and 63% of patients, respectively. The normal platelet count and LDH(Lactate Dehydrogenase) level, a biomarker for evaluating cellular damage, which are directly correlated with symptoms of PNH, decreased by an average of 80.3% from 1540.8 U/L to 303.6 U/L(doi.org/10.1111 /ejh. 13970). The normal range of LDH is 140 to 280 U/L.
In another study, blood(serum 25%) obtained from patients treated with Soliris showed an inhibitory effect on C5 activity of about 80%(doi.org/10.1182/blood-2016-08-732800). Coversin, another C5 inhibitor, showed a maximum level of 60% inhibition of C5 activity, and when Soliris and Coversin were co-administered, C5 activity was inhibited close to 100%. Coversin is a 17 kDa protein with a different binding site to Soliris and C5.
CEO Dr. Kim said, “Insufficient inhibition of C5 activity in existing drugs led to LDH levels above the normal range, which still poses difficulties in daily life. Inhibition of C5 activity in high and alternative pathways compared to competitive drugs of IM-101 is a point to expect higher therapeutic effects in patients with autoimmune diseases"
ImmunAbs is conducting a phase 1 clinical trial in the US by dividing 48 healthy clinical participants into 6 cohorts of 8 each. Each cohort consisted of 200 mg, 400 mg, 600 mg, 800 mg, 1600 mg, and 3200 mg doses of IM101(6 patients) and 2 placebo groups. ImmunAbs has now completed administration of up to Cohort 2 at a dose of 400mg, and it is encouraging that there have been no safety issues so far.
ImmunAbs expects to be able to draw the final results by the end of this year, and in the final analysis, it plans to analyze the level of free C5, a pharmacodynamic(PD) marker that represents the therapeutic effect of the drug, and proceed with follow-up clinical trials based on the results.
Myasthenia gravis as its first indication..”Effective inhibition of C5 activity compared to competing drugs in preclinical studies”
Based on the results of a phase 1 clinical trial, ImmunAbs plans to develop myasthenia gravis(gMG) as the first indication. Myasthenia gravis is a representative disease caused by neuromascular junction abnormalities and is an autoimmune disease caused by antibodies. In the early stages, symptoms such as drooping eyelids(blepharoptosis) and double vision appear, and changes in facial expression, standing, walking, and tiredness reduce basic motor skills and make daily life difficult, and sometimes death due to breathing difficulties.
Myasthenia gravis is a mechanism by which autoantibodies against acetylcholine receptors expressed on the muscle surface of the neuromuscular junction, where nerves and muscles are connected, interfere with the binding of neurotransmitters, cause the disease. At the time of diagnosis, acetylcholinesterase inhibitors are used to alleviate symptoms. In addition, Soliris, Ultorimis, and Argenx's FcRn(Neonatal Fc receptor) antibody fragment 'Vyvgart(efgartigimod)' are used as treatments.
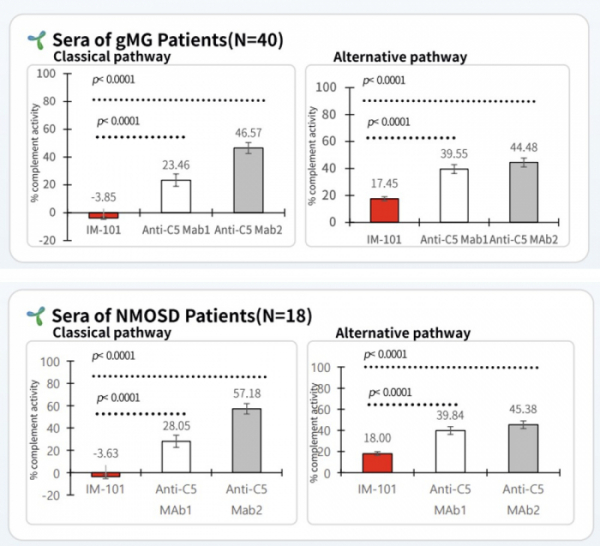
ImmunAbs analyzed the C5 activity of IM-101 and two existing drugs, C5-1 and C5-2, using blood samples obtained from 40 patients with myasthenia gravis(gMG). As a result, IM-101 significantly suppressed the C5 activity of the classical and alternative pathways compared to competing drugs(p<0.0001). The classical pathway C5 activity in the IM-101-administered group was -3.85%, and in the C5-1 and C5-2-administered groups, it was 23.46% and 46.57%, respectively. The C5 activity of the alternative pathway was 17.45% in the IM-101 group, and 39.55% and 44.48% in the C5-1 and C5-2 groups, respectively.
ImmunAbs plans to develop neuromyelitis optica spectrum disorder(NMOSD) as another indication for IM-101. NMOSD is a disease in which aquaporin-4 in the optic nerve is suppressed and the optic nerve is damaged, leading to blindness. It is known that optic nerve myelination is caused by demyelinating by the complement classical pathway, C1qrs.
ImmunAbs compared and analyzed IM-101 and two existing drugs, C5-1 and C5-2, using the blood of 18 patients with NMOSD. As a result, the classical pathway C5 activity was -3.63% in the IM-101 administration group, and 28.05% and 57.18% in the C5-1 and C5-2 administration groups, respectively. The C5 activity of the alternative pathway was 18% in the IM-101 group, and 39.84% and 45.38% in the C5-1 and C5-2 groups, respectively.
CEO Dr. Kim said, “About 10% of NMOSD patients do not respond to existing drugs, and there are side effects related to immunosuppressants, so other treatment options are needed”
In addition, ImmunAbs is also planning to develop ‘IM-201’, a candidate for diabetic retinopathy, and ‘IM-401’, a candidate for obesity treatment. Diabetic retinopathy, one of the complications of diabetes, is a disease that occurs when capillaries in the eye burst and are repeatedly formed; Mechanism of the drug candidate is to form healthy blood vessels. Candidate substances for obesity treatment are in the process of deriving candidate substances as a mechanism to increase basal metabolic rate by increasing muscle mass.
What is the status of global companies’ C5 inhibitor development?
In the field of C5 inhibitors, AstraZeneca/Alexion(AZ/Alexion)'s C5 antibodies Soliris and Ultorimis preoccupied the market, and subsequent drugs are competing. AstraZeneca signed a mega deal to acquire Alexion for $39 billion in 2020, securing various pipelines for rare diseases, including Soliris and Ultorimis. Sales of Soliris and Ultorimis last year were $3.762 billion and $1.965 billion, respectively. Soliris and Ultorimis are administered intravenously(IV) every 2 weeks and 8 weeks, respectively.
Roche showed non-inferiority in blood transfusion avoidance and hemolysis phase control in the PNH phase 3 clinical trial(NCT04434092), which directly compared the C5 antibody 'crovalimab' with Soliris in this February. Crovalimab has a different C5 binding site from Soliris and Ultorimis, and improved convenience by administering subcutaneously(SC) at 4-week intervals.
UCB significantly improved myasthenia gravis symptoms and daily living ability index MG-ADL(Myasthenia Gravis-Activities of Daily Living) score in the phase 3 clinical trial for myasthenia gravis(gMG) of the C5 target peptide 'zilucoplan' and met the primary endpoint. Zilucoflan is a drug that is administered subcutaneously(SC) every day. Currently, the US Food and Drug Administration(FDA) has approved a new drug application(NDA) and is reviewing whether to approve it or not.
UCB acquired Ra Pharma for $2.1 billion last year and added zilucoplan and subcutaneous FcRn antibody candidate 'rozanolixizumab' to its pipeline. UCB received approval from the FDA in January of this year for a biopharmaceutical license application(BLA) for rozanolixizumab for myasthenia gravis and is under review through a priority review process.
In February, Regeneron received priority review from the FDA for its C5 antibody 'pozelimab' for CHAPLE disease, an ultra-rare hereditary immune disease. Pozelimab is scheduled to be approved by the FDA by August 20, and if approved, it will be the first drug for CHAPLE disease. CHAPLE disease is a genetic disease caused by overactivation of the complement system due to a deficiency of CD55, which regulates the complement system, and is known to have fewer than 100 patients worldwide.
Regeneron, along with Alnylam Pharmaceuticals, is conducting a phase 3 clinical trial for the combination therapy of pozelimab and C5 RNAi 'cemdisiran' for myasthenia gravis and paroxysmal nocturnal hemoglobinuria.

















