탐방
Precision Biosensor targets the POCT market with highly sensitive TRF
by Joungmin Cho
Possess one-stop total diagnostic solution and develop highly sensitive Point-of-Care-Testing diagnosis: develop next-generation products and advance to global market in earnest by securing TRF source
▲CEO Kim Hanshin, Precision biosenser.
Precision Biosensor was set up in 2015 through the merger between Nano Ditech, a diagnostic reagent company established in 2002, and TERAWAVE, an analytical and diagnostic device company set up in 2009 as an in-house venture of the KAIST. It is now developing an immunodiagnosis platform for highly sensitive and functional Point-of-Care-Testing (POCT).
The company develops time-resolved fluorescence diagnosis technology using Europium, which exhibits one million times higher luminous efficacy than ordinary fluorescence, and carries various diagnostic products based on this technology. The self-developed analytical device known as 'Exdia' can be used not only for disease diagnosis but also monitoring disease progression.
Precision Biosensor has successfully attracted an investment equivalent to 11 billion won from five institutions including Korea Investment & Securities, Atinum Investment, DSC Investment, Seoul Investment Partners, and I-Sense.
◇ Diagnostic Reagent (Nano Ditech) + Analysis Device (Tera Wave) -> One-stop Total Solution (launch of Precision Biosensor)
The company has transformed itself into a total-solution company that deals with a wide array of businesses ranging from the supply of raw materials to the development, manufacturing, distribution and sales of diagnostic reagents and testing devices. "There are only five companies that are doing businesses in both reagents and diagnostics markets among domestic diagnostics companies," says the CEO, Kim Hanshin.
Precision Biosensor has sought changes to improve the fundamentals of the company and to secure a growth engine. The company has evolved from an Original Equipment Manufacturer (OEM) and Original Development Manufacturer (ODM)( to its own brand to challenge the self-development of highly sensitive, immune testing cartridges, and test devices.
CEO Kim says: "When I was focusing on changing trends in the in-vitro diagnostics market, I thought there would be an opportunity. I found a paradigm shift from a simultaneous large-scale diagnosis to POCT diagnosis." According to Kim, there is an increased demand for early and rapid diagnostics of cardiovascular diseases, and for immune tests that perform detection and diagnosis in field sites to prevent the spread of infectious diseases including tuberculosis and Ebola.
Precision Biosensor has applied for patent applications in several countries in the EU, the US, and South Korea, for its medical devices including ‘Nano-checker’, ‘Fluoro-checker’, and ‘Exdia TRF.'
"In addition to the existing products that produce fixed incomes, our expansion of product lineup with various products and our overseas sales network into new markets including Europe are expected to drive the company's growth in full swing starting from 2020,” according to the CEO, Kim Hanshin.
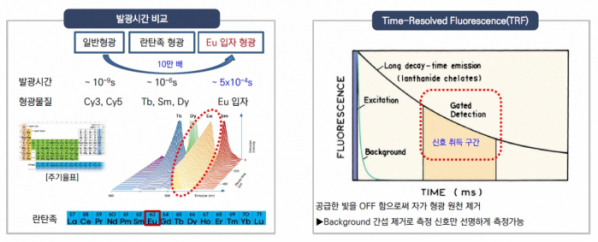
◇ Developed time-resolved fluorescence technology, which utilizes Europium with luminous efficacy 100,000-fold higher than ordinary fluorescence
Especially, Precision Biosensor is particularly focused on time-resolved fluorescence (TRF) technology that is used to increase the sensitivity of fluorescence signals emitted while reducing the noise that often occurs when ordinary methods of fluorescence measurement are used. Many diagnostics companies participated in the development because they saw great potential in TRF technology, and are now at a dead-end due to technical barriers.
Precision Biosensor utilizes 'Europium' with an extended luminosity and a wide reach among lanthanides. Europium can glow 100,000 times longer than ordinary fluorescence. According to the company, "non-specific signals can be removed by utilizing the extended glow property of Europium, and it is the most optimized material for the TRF technology, which maximizes the sensitivity for signal detection.”
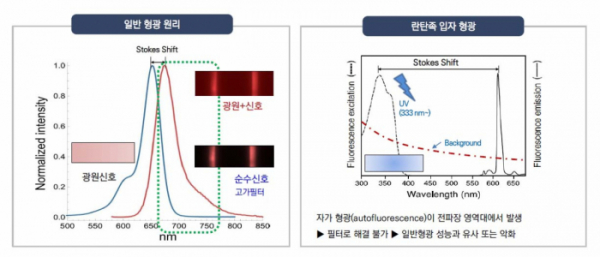
CEO Kim says: "The existing diagnostic products can detect nanograms (ng) of biomarkers present in the blood, and Precision Biosensor's TRF diagnostics using Europium has a high sensitivity that can detect picograms (pg) of biomarkers, which are 1,000 times less than nanograms."
Also, Precision Biosensor's TRF diagnostic products are applied with an algorithm that converts signals into images, for quantitative analysis, instead of simple detection of signals.
'Exdia' an imaging TRF testing device developed inhouse by Precision Biosensor, can realize a uniform light source and detect signal light simultaneously. It calibrates Europium signals and processes the acquired signals into images using an artificial intelligence algorithm. CEO Kim commented: "As it uses satellite scan technology, it can read the entire kit with a single scan instead of reading part by part. This is how it can show high sensitivity and accuracy even when a cheap filter is used.
The cartridge used for Exdia is a collection of Precision Biosensor's knowhow. Based on the understanding of protein targets, it produces high-performance raw materials via development and production of antigens and antibodies, and applies the best optimized reagent.
Precision Biosensor has completed the development of a total of 10 POCT products including five kinds of diagnostic products for cardiovascular diseases, which require emergency diagnosis and other diagnostic products for detection of occult blood in fecal samples, pregnancy, and influenza infection.
◇ In cooperation with a global NGO, it is developing a tuberculosis diagnostic product. The TB blood diagnostic product using Aptamer is planned to be commercialized by 2021
After establishing its technological competitiveness, Precision Biosensor is conducting various collaborative research projects on a global stage, and the most representative project is the development of a blood-based diagnostic product for tuberculosis that was launched in 2017 in cooperation with 'FIND,' a global non-profit organization.
"Our short-term goal is to strengthen our expertise in the immune diagnostic field and to expand our sales network to new markets including Europe and the U.S. After that, our long-term goal is to produce a biomarker platform that can be used for new drug development and efficacy verification, through diversification of our diagnostic field," explained CEO Kim.



















