탐방
G+FLAS Challenges Anti-Cancer Drug Based on ‘CRISPR PLUS’ Technology
by Euna Lee
G+FLAS Life Science, which recently raised funds of 14 billion KRW with Series B, is now spurring on the development of anti-cancer drugs based on CRISPR PLUS technology. The company secured the original CRISPR technology last year from the successful funding of 4.2 billion KRW with Series A, and then committed for the development of CRISPR PLUS technology, which is an upgrade version of the original CRISPR technology. This year, the company intends to develop bio-medicine that exploits the CRISPR technologies.
Choe Sunghwa, the President and CEO of G+FLAS Life Science, told the reporter from Bio-Spectator at the headquarters of the company in the Nakseongdae R&D Center located in Gwanak-gu, Seoul, “we will introduce new techniques of applying therapeutic agents based on core capabilities of CRISPR technology. Plant-based antibody biologics are advanced to go beyond the limit of the current CHO-based animal system for developing antibodies customized for individuals. In addition, the CRISPR technology, which is able to discern cancer mutations out of wild-type sequences, will be exploited for the development of anticancer agents that features the CRISPR tools themselves as drugs; this will lead us to a path to become one of the leading companies in the application of CRISPR technology to human therapeutics and personalized medicine”

▲최성화 지플러스생명과학 대표
From CRISPR PLUS to “CRISPR ANTI-CANCER”
G+FLAS Life Science has developed the CRISPR PLUS, which resulted from an enhancement of the performance of the existing CRISPR technology. The CRISPR PLUS technology includes CRISPR-Cas9 and CRISPR-Cas12, which are prepared to correct DNAs, and CRISPR-Cas13, etc. that likewise correct RNAs. For the patent protection of the CRISPR PLUS technology, G+FLAS Life Science has filed patent applications for CRISPR PLUS in Korea as well as in the U.S.A., together with patent applications for non-GMO plant seeds resulted from the technology and the anticancer agent(s) developed therefrom, for which part of the patent examinations are currently in progress.
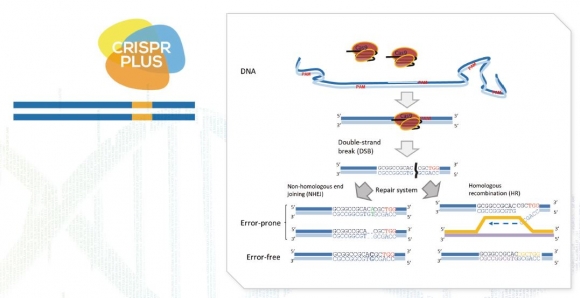
▲지플러스생명과학의 '크리스퍼플러스(CRISPR PLUS)' 기술 모식도 (그림: 회사 제공)
G+FLAS Life Science has ambitiously prepared the “CRISPR Anti-Cancer Agent(s)”. Developmental efforts are currently focused on transforming CRISPR Tools into Anti-Cancer Agent(s). G+FLAS Life Science’s approach is different from other conventional approaches that are intended for the restoration of mutant genes through transforming them into wild-type genes, by correcting them using CRISPR technology. G+FLAS Life Science’s approach uses the apoptosis of cancer cells by cutting mutant genes in cancer cells instead.
CEO Choe also commented, “CRISPR Anti-Cancer Agent achieves anticancer effect from the cut-off of genes enabled by the ability of CRISPR to accurately locate specified genes. The concept of “CRISPR Anti-Cancer Agent” can bypass current controversial safety issues. The only problem is the possibility that CRISPR may unpredictably edit non-targeted DNA in cells. But these possibilities will not be a stumbling block, since the CRISPR Anti-Cancer Agent was designed to destroy cancer cells once the target DNA was cut-off. The concept of cut-off of mutant genes to kill cancer cells will let it overcome situations involving resistance, as well.”
There are additional advantages to the use of CRISPR Anti-Cancer Agent. Even the ‘undruggable’ therapeutic targets can be turned into druggable, since the crRNA which guides CRISPR-Cas enzymes to target genes rather than gene products. He emphasized that “we will ultimately realize the customized precision medicine based on the CRISPR technology. Patients’ tissues are to be sequenced to design customized CRISPR Anti-Cancer Agent that is enabled to locate mutated genes in the cancer cells.”
G+FLAS Life Science developed a plan to construct a domestic-overseas network to be dedicated to the research and development of CRISPR Anti-Cancer Agent. For this, 100 % share of an American corporation, Naturegenic, was acquired to secure the base of the network. Naturegenic, originally founded by President Choe in September 2014 as a company for crop improvement based on CRISPR technology, is located in the Research Park of Purdue University, U.S.A.
In addition, G+FLAS Life Sciences is currently developing the ‘GF003’, the plant-based antibody medicine, as a front pipeline. GF003 is a Herceptin Biobetter, of which the antibody-dependent cellular cytotoxicity (ADCC) was improved by CRISPR technology that improved the glycol-pattern of the expressed proteins in the host plants to reduce immunogenicity and efficacy of the biobetter.

▲지플러스생명과학은 크리스퍼기술을 활용해 식물기반 항체의약품을 개발하고 있다. 지플러스생명과학 실험실 사진
Currently, G+FLAS Life Science completed the correction of glycosylation patterns of plants through the application of CRISPR PLUS technology; the nonclinical trials thereof will begin this year.
G+FLAS Life Science will also construct small-scale GMP production facilities for the plant-based biologics in the 4.1 acre campus located in Osong. G+FLAS Life Science also recruited talented scientists from the National Cancer Institute (NCI) and domestic CROs for the development of GF003 and anticancer drugs.
President Choe concluded the interview with the following comment, “The business objective of this year is to generate sales from our products. Based on the CRISPR technology of G+FLAS Life Science, sales are expected that will be realized from the development of crops and CAS9 antibodies. Licensing-out of an improved version of the existing technology, the CRISPR PLUS technology, is also in progress. We are working tirelessly with goals of having the company listed on KOSDAQ in 2020.”